Refractive Index Increments to Calculate the Refractive Index of a Single Solute in a Buffer Solution: An Experimental Course
Abstract
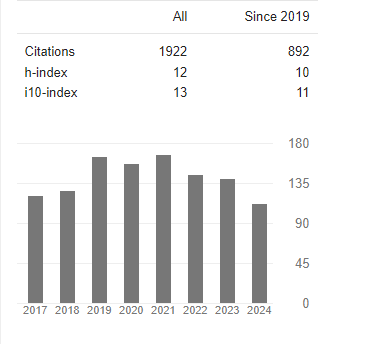
Vincent BALL
An experimental protocol for BSc students is proposed in which the refractive index increment at the wavelength corresponding to the sodium D line (λ=589 nm) of solutions containing different isomers of a solute is proposed. The used molecules are the isomers of dihydroxy benzene at pH = 5, namely 1,2-dihydroxybenzene (catechol), 1,3-dihydroxybenzene (resorcinol) and 1,4-dihydroxybenzene (hydroquinone). But in principle the experiments could performed with other isomers of a molecule in a suitable solvent ensuring perfect solubility of the chosen solute. The obtained refractive index increments are then used to calculate the refractive index of the solute (at this particular wavelength) knowing its density. The results are compared with the literature data and related to molecular properties like the polarizability and dipole moment. Error calculation are proposed followed by a discussion about statistical differences or not between the refractive index of the three investigated substances.