Research Article - (2023) Volume 8, Issue 5
The Discovery of Menstrual Derived Stem Cells and their Therapeutic Effects
Received Date: May 08, 2023 / Accepted Date: May 13, 2023 / Published Date: May 24, 2023
Abstract
Stem cell therapy is the future of medicine as research is ever-growing for uncurable diseases and injuries. Stem cells are difficult to obtain due to ethical conflicts, retrieval difficulties, or high expenses. However, a stem cell line referred to as menstrual derived stem cells (MenSCs) show great potential in addressing all these issues. MenSCs are a type of adult stem cell that can be found in menstrual blood. These cells are considered a valuable source of stem cells due to their ease of accessibility, abundance, and lack of ethical concerns. MenSCs have been found to possess properties similar to other adult stem cells, such as the ability to differentiate into various cell types, including nerve cells, bone cells, and cartilage cells.
Research has shown that MenSCs have the potential to be used in regenerative medicine for various conditions, including cardiovascular disease, nervous system repair, spinal cord injuries, liver and lung health, as well as skin repair. Additionally, MenSCs have been found to possess anti-inflammatory and immunomodulatory properties, making them a potential candidate for the treatment of autoimmune diseases. Despite the potential benefits of MenSCs, further research is needed to fully understand their properties and potential uses. Currently, there are ongoing clinical trials to evaluate the safety and efficacy of MenSCs for various conditions.
Keywords
Alzheimer’s Disease, Endometrial Mesenchymal Stem Cell, Parkinson’s Disease, Premature Ovarian Failure, Broncho Alveolar Lavage Fluid
Abbreviations
Aβ: Amyloid-Beta; AD: Alzheimer’s Disease; AKT: Protein Kinase B; ALI: Acute Lung Injury; ALT: Alanine Transaminase; AST: Aspartate Aminotransferase; BALF: Broncho Alveolar Lavage Fluid; bmMSCs: Bone-Marrow Derived Mesenchymal Stem Eell; bFGF: Basic Fibroblast Growth Factor; DAM: Diacetylmorphine; DMD: Duchenne Muscular Dystrophy; ECM: Extracellular Matrix; eMSC: Endometrial Mesenchymal Stem Cell; ESCs: Embryonic Stem Cells; FAK: Focal Adhesion Kinase; IC: Intracerebrally; IV: Intravenously; iPSCS: Induced Pluripotent Stem Cells; KEGG: Kyoto Encyclopedia Of Genes And Genomes; LPS: Lipopolysaccharide; MenSCs: MenstrualDerived Stem Cells; MoDCs: Monocyte-Derived Dendritic Cells; MPO: Myeloperodixase; MSC: Mesenchymal Stem Cell; NIDDM: Non-Insulin Dependent Diabetes; NK: Natural Killer; PBMC: Peripheral Blood Mononuclear Cells; PCR: Polymerase Chain Reaction; PD: Parkinson’s Disease; PF: Pulmonary Fibrosis; POF: Premature Ovarian Failure; SCI: Spinal Cord Injury; TA cells: Transit Amplifying Cells; TGF-β: Transforming Growth Factor Beta; VEGF: Vascular Endothelial Growth Factor.
Introduction
Regenerative medicine is an ever-growing field useful in treating/ investigating a variety of diseases and at the root of this field is stem cell therapy. Stem cells are undifferentiated cells that have the ability to self-renew and differentiate into various cell types. They can be considered the “building blocks” of the human body as every cell with a specialized function originates from this. They have been extensively studied for their therapeutic potential in regenerative medicine, as well as for their immunomodulatory and anti-inflammatory properties [1].
Stem cells can be classified from their differentiation potential based on their origin and derivation into five groups: totipotent, pluripotent, multipotent, oligopotent, and unipotent cells. Totipotent cells are found in the early stages of life as they play a role in forming the embryo and placenta. Pluripotent cells differentiate into the three germ layers that arise from development: the ectoderm, endoderm, and mesoderm. Multipotent stem cells are present in most tissues and have the ability to differentiate into cells from a specific germ layer as seen in the bone marrow, umbilical cord blood, peripheral blood, etc. Oligopotent cells are able to form two or more lineages within a specific tissue and unipotent cells give rise to a single lineage and differentiate into one specific cell type [2] (Figure 1).
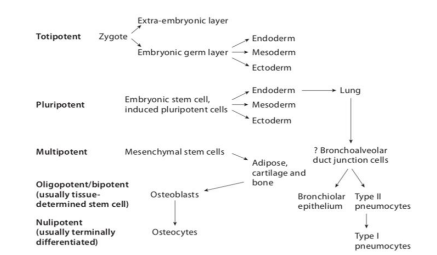
Figure 1: Displays the hierarchy of stem cells [2].
| Differentiation potential | Origin |
|---|---|
| Totipotent or omnipotent | |
| Pluripotent | ESCs, iPSCs |
| Multipotent | Fetal stem cells |
| Oligopotent | Adult or somatic stem cells |
| Unipotent |
Table 1: Displays stem cells classification based on differentiation potential and origin.
ESCs are embryonic stem cells and iPSCs are induced pluripotent stem cells [2].
However, harvesting stem cells has its own challenges. For example, embryonic stem cells give rise to all tissues and organs but obtaining them poses an ethical dilemma as one would have to destroy a human embryo. Adult stem cells pose no ethical risk but cannot be easily manipulated into specific cell types and may need invasive procedures for collection [3]. Essentially, the current challenge is finding stem cells that are easily obtained,pose no ethical conflicts, affordable, and unlimited.
One promising source of stem cells is menstrual blood, which contains a population of stem cells known as menstrual-derived stem cells [MenSCs]. First reported in 2007, these adult stem cells are multipotent and can differentiate into a variety of cell types, including adipocytes, osteoblasts, chondrocytes, and endothelial cells [4].
Lining the human endometrium of the uterus is endometrial mucosal lining, a highly regenerative tissue located on muscular myometrium and originates from the Müllerian ducts during embryonic development. The human endometrium remarkably regenerates monthly, increasing its thickness from 0.5-1 mm after menstruation back to 5-7 mm during each menstrual cycle [9]. Studies indicate that the endometrium contains a population of cells similar to stem cells obtained from the bone marrow based on pluripotent differentiation and self-renewal abilities [5]. These particular cells can be located in the basalis endometrium rather than the functionalis or myometrium layer as seen in Figure 2 and give rise to proliferating TA cells in the functionalis layer.
Studies on primates and clinical practice provide indirect evidence for the presence of MenSCs after complete regeneration of the endometrium following majority surgical resection of the endometrium that can support pregnancy. In another clinical situation, women experiencing menorrhagia [excessive bleeding] have a procedure referred to as electrosurgical ablation which uses an electrical current to remove the endometrial lining of the uterus to reduce the symptoms. Despite the removal, pockets of endometrial tissue are seen to regenerate [9].
During the menstrual cycle, angiogenesis is critical in the development of uterine lining as it creates new blood vessels from existing vasculature which can lead one to believe that progenitor/stem cells are involved in this process, and it may be possible to obtain stem-like cells from the shedding endometrial tissue. In an experiment led by Dr. Amit Patel, he isolated a population of stromal cells from menstrual blood and observed self-renewal and differentiation abilities, demonstrating stem cell capabilities [6]. Further supporting the idea of stem cell evidence in menstrual blood lies in the condition of endometriosis. This is a condition describing endometrial tissue being present outside the uterus occurring with or without the menstrual cycle. Sampson’s reflux implantation theory is said to explain the cause of this, stating that endometrial stroma/debris flow backwards via fallopian tubes and reach the abdominal cavity where they implant and grow. Because stem cells are thought to originate from the shedding of the endometrial lining, the progression of endometriosis indicates evidence of stem cell development [7].
Research also suggests that during the menstrual cycle, a small percentage of endometrial cells may enter circulation and aid in the natural regeneration of vital organs in the body, providing a protective barrier for women during their reproductive years. This can be confirmed in higher rates of obesity, myocardial infarction, osteoporosis, NIDDM, and hypertension present in post-menopausal women, suggesting the risk is evident of a lack of replenishment from these stem/progenitor cells [8].
Originating from the endometrial lining of the uterus, MenSCs undergo periodic regeneration in menstruating women under hormonal control; thus, giving rise to a robust population of stem cells that renew every month (Figures 2 and 3).
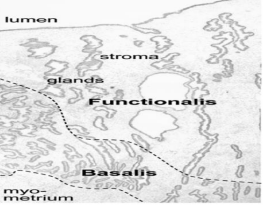
Figure 2: Displays a diagram of the human endometrium. The endometrium is divided into two major zones: the basalis and the functionalis. As seen, there are glands coming from the surface epithelium extending into the functionalis which is loosely held together by the stroma and lower basalis, which consists of denser stroma and lymphoid aggregates [9].
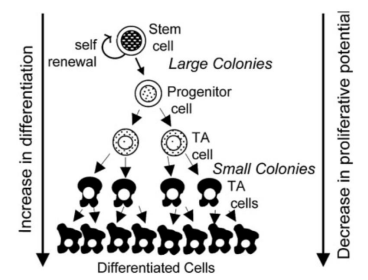
Figure 3: Displays a hierarchy of stem cell differentiation, where the cells go through asymmetrical cellular division, enabling selfreplication or differentiation eventually to TA cells [transit amplifying cells] which lead to specialized cells. The possible colonial relationship initiated by endometrial epithelial cells and stromal cells is also demonstrated [9].
The biological characteristics is a significant point of interest for MenSCs. A study was conducted by Xinxiang Medical University to localize these cells, investigate the duration of menstrual blood storage prior to isolation, age of female donors, the number of passages affecting MenSC quality, and other organic attributes following the transfer of MenSCs to another organism. Menstrual blood samples were obtained from healthy female donors during the first few days of menstruation (collected via menstrual cups) and promptly mixed/stored with a phosphate-buffered saline solution before being delivered to their laboratory within seventy-two hours. It was noted that the MenSCs were durable for at least three days under 4°C [10] (Figure 4).
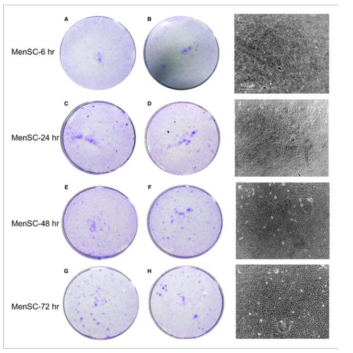
Figure 4: Shows the storage duration impact on MenSCs viability prior to isolation. A-B show MenSC isolation after six hours, C-D after 24 hours, E-F after 48 hours and G-H after 72 hours. As one can observe, there was no significant difference
The karyocytes and endometrium were isolated through centrifugation and suspended in a growth medium. The cells were then seeded into cell culture flasks to incubate for adherent cells that grew as fibroblastic cells. The medium was replaced every three days until the culture reached 80-90% confluence where they were detached and subcultured into new flasks at a 1:3 ratio. The MenSCs were identified by their radioactive/helix growth pattern in the first-generation culture period, overtaking the nonadherent cells. There was a spindle, fibroblast-like structure after passaging for the whole culture period in vitro. This was compared to the MenSCs from the third generation and adult stem markers, CD29, CD44, CD73, CD90, CD105, and HLA-ABC were expressed. The MenSCs also displayed markers associated with embryonic stem cells, SOX2, c-MYC, SSEA-4, OCT-4, and Nanog. Expected differentiation occurred (neurogenic, adipogenic, chondrogenic, cardiogenic, and osteogenic differentiation) after being cultured in a differentiation medium to test for pluripotency [10] (Figure 5).
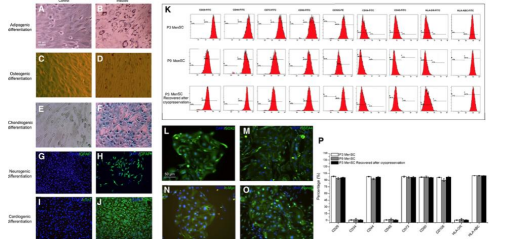
Figure 5: Displays the identification of the MenSCs. A-F display osteogenic, adipogenic, and chondogenic differentiation. G-J demonstrate cardiogenic and neurogenic differentiation while K displays the immunophenotype observed with P3 and P9 (different passage stages) MenSCs expressing the ASCs markers mentioned previously (CD29, CD44, CD73, etc). L-O express the ESC markers mentioned previously (c-MYC, Nanog, etc) and P displays the flow cytometry quantification [10].
To assess the proliferative capacity of the different passages of MenSCs, the cells were obtained from three age groups: twenty, thirty, and forty years old. Cells maintained at various passages (P3, P9, and P18) were seeded at different densities. Passages refers to the number of times the cells had been cultured and divided before being evaluated. An MTT assay was used to measure the metabolic activity and indicate viability/proliferation. It was noted that the older the donor, the slower the proliferation rate of the cells, with a noticeable difference being seen in the jump from P9 to P18 [10] (Figure 6).
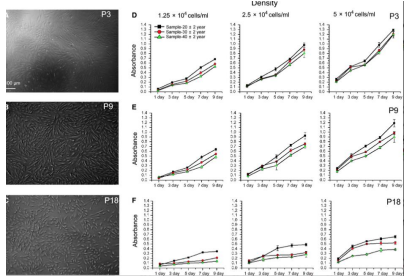
Figure 6: Displays how donor age and passage number affect the proliferative capacity of MenSCs. A-C show the structure of the different passages, P3, P9, and P18. D-F show the MTT assay results from the passages being cultured at the indicated ages over a span of nine days [10].
Immunogenicity was evaluated by transferring MenSCs intravenously or intraperitoneally to mice and no adverse effects occurred on their immune systems as one can see in Table 2.
Studies also indicated that MenSCs supposedly have lower tumorgenicity compared to ESCs. In order to test this, researchers injected a tumor cell line called pheochromocytoma and MenSCs in immunodeficient mice with results indicating no tumor development in mice injected with MenSCs [11] (Figure 7).
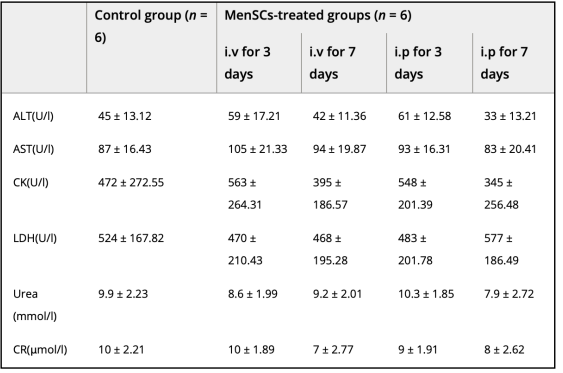
Table 2: Displays content of urea, pathology-related enzyme, and creatine activity following transplantation at three and seven days [10]
Other studies can confirm the methods and results conducted by Xinxiang Medical University and add rapid propagation time to be around 18-36 hours with similar morphology [12]. However, MenSCs appear to have a shorter lifespan compared to human embryonic stem cells but appear to have a higher telomerase activity which can be deduced to the constant regeneration of the endometrium during the cycle of menstruation [6].
In addition to the immunomodulatory characteristics mentioned in the experiment, MenSCs were compared to bmMSCs (bonemarrow derived mesenchymal stem cells) to study T cell responses in mixed lymphocyte reactions where MenSCs were mixed with allogenic human peripheral blood mononuclear cells (PBMC). MenSCs were observed to have distinct immunosuppressive activity as seen in suppressed cellular proliferation, IFN-y/TNF levels, and increased IL-4 production [13]. It is also a point of interest to note that this suppressive effect is dependent on dose and concentration of MenSCs with PBMC proliferation being suppressed by a high MenSC:PBMC ratio [14]. This can also be seen in reduced anti-inflammatory IL4+IL10+CD4+ T cells where cytokine levels were MenSC concentration dependent but at a lower MenSC:PBMC ratio compared to bmMSC [15].
MenSCs also appear to affect the innate immune system or specifically the natural killer cells (NK), monocyte-derived dendritic cells (MoDCs) and tissue macrophages. T cell activation requires MoDC differentiation and expression of co-stimulatory molecules, but MenSCs interfere with these processes, thus inhibiting T cell activation. In the presence of pro-inflammatory cytokines such as IFN-y/IL-1β, NK proliferation and cytotoxicity is inhibited by MenSCs production of IL-6 and TGF-β while proliferation can be induced without the inflammatory cytokines by the MenSCs. Also, along with the maintenance of endometrial tissues homeostasis, a pregnancy-friendly phenotype in decidual NK cells may also be retained by MenSCs which is important for a successful pregnancy [16]. Thus, one can conclude that the immunomodulatory responses of MenSCs are significant enough to use for therapeutic applications in organ transplantation, autoimmune-related diseases, etc. Antimicrobial activity can also be characteristic of MenSCs as seen in a study investigating the effects on a sepsis-induce mouse model. MenSCs were combined with antibiotics for sepsis treatment and increased survival rate was observed compared to treatment of just the antibiotic or cells alone. Bacteria clearing and reduced inflammation was also encouraged by the combination treatment without causing a loss of T and B lymphocytes [17] (Figure 7).
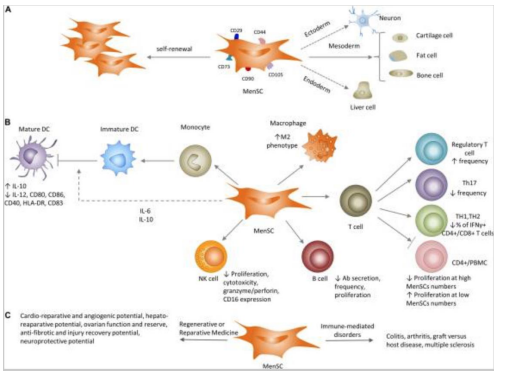
Figure 7: Refers to MenSCs characteristics in vivo and in vitro. A shows MenSC differentiation abilities leading to MSC marker expression. B shows the impact of MenSCs on innate and adaptive immune systems. C refers to the application of MenSCs in preclinical settings for immune-mediate disorders [18].
Discussion
Because of the properties studied on MenSCs, these stem cells show promising potential for clinical application, making them a favorable cellular source. This discussion will briefly discuss its impact on various clinical uses. MenSCs have been shown to have potential benefits for cardiac health with research targeting cardiac diseases and damage. One experiment in particular was investigating the use of MenSCs in improving heart function after a myocardial infarction. MenSCs were transplanted into replicated heart-attack rat models and improvements were seen in the function of the left ventricle of the heart. In addition, fibrotic tissue (scar tissue) was shown to be reduced following the transplantation [19]. A similar study on myocardial infarctions was investigated MenSC differentiation into cardiomyocytes. The stem cells were injected into ischemic zones of an immunocompetent rat model showing symptoms of a heart attack and results indicated successful differentiation of MenSCs into heart muscle cells without fusion of the donor and recipient cells. MenSCs are also seen in murine models treating Duchenne muscular dystrophy (DMD), a condition that can lead to cardiomyopathy. Implanted MenSCs were seen promoting cell fusion of host myocytes and implanted cells to restore the normal levels of muscular dystrophin, leading to increased muscle strength. This can be seen in vitro with similar results occurring through cell fusion and transdifferentiation [21]. The paracrine characteristics of MenSCs can be seen in a study by Jiang et. al where xenogeneic transplantation was performed to overall improve heart function. Cytokine secretion was discussed previously as a way to help with organ regeneration, and paracrine effects refer to MenSCs stimulating this as well as preserving the myocardium by activating survival pathways and recruiting endogenous progenitor stem cells through the secretion of cytokines [22]. In addition, MenSCs B-cell suppression and inhibition of the humoral response can lead to decreased severity of antibody-mediated allograft rejection. MenSC transplantation led by a research group led to improved cardiac function and a prolonged survival of a cardiac allograft [23]. As one can see, various studies show overwhelming results in MenSCs improving cardiac function in animal models of heart attacks and heart damage. MenSCs show great potential in offering therapeutic approaches for the treatment of heart disease (Figure 8).
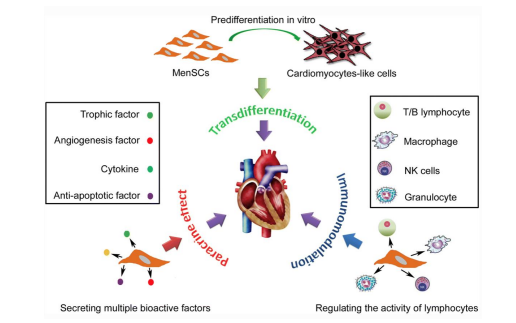
Figure 8: Shows potential mechanisms of MenSCs such as its paracrine effects and immunomodulation in cardiac disease treatment to promote regeneration and improved function [24].
MenSCs show great promise in the treatment of neurological/ neurodegenerative disorders and can potentially differentiate into glia/neural cells in vitro. In cases of stroke in adult rat models, MenSCs have potential to improve motor and behavioral symptoms as well as reduce neuronal death when intracerebrally or intravenously transplanted. For example, a study observed the survival of transplanted MenSCs in the penumbra, an area of brain tissue surrounding the core stroke damage that was deprived of blood flow and oxygen. The cells were able to maintain their presence and expressed a distinct eMSC marker, OCT4, leading investigators to believe that specific growth factor secretions may play a role in the maintenance of endogenous neural cells over cell replacement [25]. Another study investigated the neuroprotection effects after a stroke where oxygen glucose deprived rat neurons were exposed to MenSCs. Results indicated that the neurons combined/exposed to the stem cells shows a significant decrease in cell death because of trophic factors secreted by the stem cells. MenSCs were also transplanted in ischemic stroke-induced adult rats without immunosuppression and found behavioral and histological impairments to be significantly reduced as well as increase neuronal cells compared to the control group 26]. MenSCs also have potential in treatment of Alzheimer’s disease (AD). AD is thought to be brought upon by the coagulation of beta-amyloid within the brain so in an animal model of AD, MenSC transplantation is shown to reduce the accumulation and improve cognitive function. Results also included survival/growth promotion of neural cells and reduced neuroinflammation in the brain [27]. MenSCs can also be used in the treatment of Parkinson’s disease (PD), an illness mainly caused by the reduction of dopamine levels in the striatum as a result of dopamine neuron degeneration in the substantia nigra of the brain. Dopaminergic neurons were generated by endometrial stem cells and transplanted into a PD mouse model. The cells showed evidence of differentiation into nerve axons in vitro and expressed carious neural markers. This engraftment led scientists to conclude MenSCs can rectify behavior disorders and exert neuroprotection because the neurons appear to survive and form functional connections in the brain [28]. Nerve repair and treatment is another disease that can also receive the benefits of MenSCs. MenSC-seeded-gelatin-based scaffolds were considered in a study as potential treatment for sciatic nerve defects in rats. Results indicated improved sciatic nerve function and reduced muscle loss following implantation of the scaffold. This is comparable to the standard treatment of using an autologous resected nerve segment in order to bridge the nerve defect, suggesting MenSC scaffolds as a cheaper and easier alternative for sciatic nerve defects [29]. Another challenge to nervous system repair is a traumatic spinal cord injury (SCI) which is highly resistant to regeneration. However, apoptosis inhibition, axonal regeneration, and myelination activation can be promoted by MenSc transplantation. This promotion can come from differentiation, neurotrophic factor production, and immune modulatory activites [30]. In a study led by Wu t. al, the expression of key neuronal ad glial scar markers was investigated. MenSCs were transplanted into a rat model with a spinal cord injury and locomotor recovery and the microenvironment at the site of the injury were observed after four weeks of transplantation. There was a significant increase in glial scar markers seven days after transplantation meaning glial scar formation could be reduced, giving way to axonal sprouting. Scar formation is a barrier to improved axonal growth so this could allow axonal regeneration to be promoted after a spinal cord injury [31] (Figures 9-12).
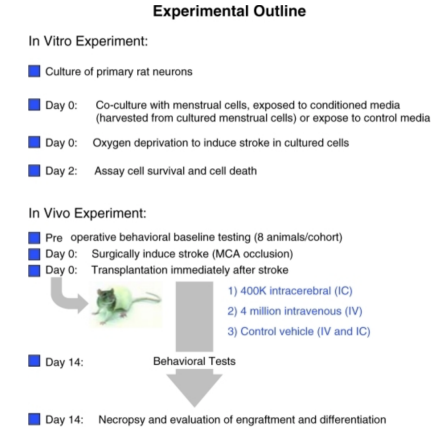
Figure 9: Shows schematic diagrams of in vitro and in vivo stroke experiments [25].
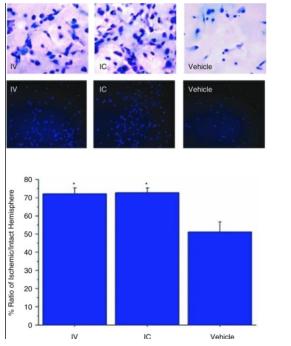
Figure 10: Shows evidence and pictures of increased evidence of host cell survival following transplantation of IC or IV transplantation of MenSCs [25]. Figure 10: Shows evidence and pictures of increased evidence of host cell survival following transplantator
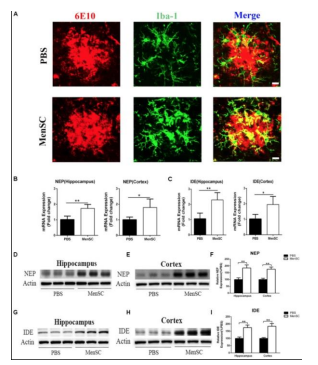
Figure 11: Shows recruit of microglia following MenSCs transplantation to cluster around Aβ plaques, leading to microglial clearance capacity restoration [27]
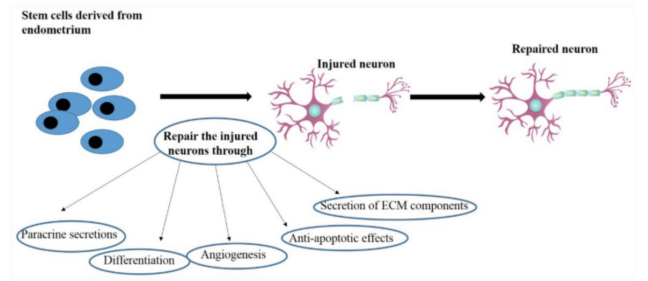
Figure 12: Illustrates the potential healing mechanisms MenSCs in nervous system repair [32].
Therapeutic effects can be seen in the treatment of the human liver. An in vitro study was conducted to investigate MenSC potential in the treatment of liver disorders such as cirrhosis and acute liver failure. Once MenSCs were isolated, researchers cultured them in the presence of growth factors and cytokines that could encourage differentiation into hepatic cells. PCR (polymerase chain reaction), immunocytochemistry, flow cytometry, and observation of hepatic-specific cell marker expression was used to confirm the differentiation. Findings indicated that MenSCs were able to differentiate into hepatic cells as they expressed markers such as CK18, AFP, and ALB, all markers that are expressed on mature hepatocytes. Based on these observations, researchers were able to hypothesize that MenSCs could be used as potential therapy for liver disorders [33]. An in vivo experiment was conducted in mice models to test MenSCs for their ability to treat acute liver failure. Within two hours of transplantation, MenSCs had localized in the damaged liver and improved liver function/histology within weeks of the injection. A point to note is the reduction of serum levels of live enzymes and metabolites, such as ALT, total bilirubin, AST, and urea, in the mice. The reparative and protective role of MenSCs after its injection was also seen in decreased levels of collagen fiber deposition, inflammatory cell infiltrate, hepatic degeneration, and improved glycogen storage, providing further evidence of the stem cells’ effectiveness in liver disorder treatment [34]. MenSC paracrine activity is said to be the likely culprit for improved function as well which means cells’ secretions are more significant than their differentiation. In fact, a few MenSCs differentiated into hepatocytes in a mouse model with liver fibrosis suggesting that the environment may effect its ability to differentiate. Granted, there was an improvement in overall function but end-stage fibrosis, specifically, may not encourage survival of MenSCs. Thus, it brings up the questions whether regeneration via MenSCs is affected by a complex, diseased environment or whether there are simply more secretions over cell differentiations that lead to improved function [35] (Figure 13).
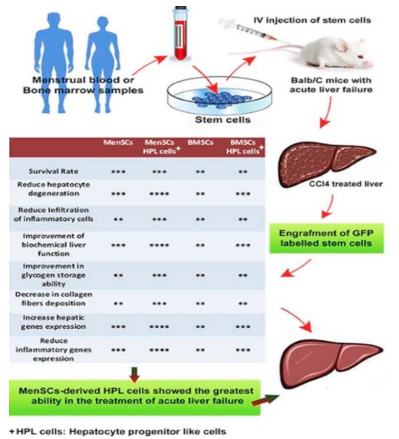
Figure 13: Shows immunohistochemical analysis of the liver following MenSCs transplantation/injection and indicate its successful ability to improve liver function [34].
MenSCs are seen to be used in a reparative capacity in the treatment of premature ovarian failure (POF) in rat/mouse models. In a study conducted to restore ovarian function, MenSCs were injected into the ovaries or bloodstream in an attempt to improve function and reduce apoptosis. Following injection, ovarian markers such as, Amh, Vegfa, Fshr, inhibin âº/β, and Ddx4 were observed to be in increased levels. Plasma levels of progesterone and estrogen are key hormones in the regulation of the menstrual cycle and pregnancy maintenance and there was noted to be a rise in these hormones. Furthermore, the number of primary, mature, and total ovarian follicles were increased after the injection [36]. MenSC differentiation into ovarian cells was investigated in another research where the stem cells were transplanted into the ovaries of mice. mRNA expression patterns were compared via microarray analysis before and after the transplantation. The analysis revealed that the expression patterns of the ovarian cells after transplantation were quite similar to human ovarian tissue compared to the non-transplantation patterns. This can lead one to assume that the MenSCs differentiated successfully into ovarian cells or fused with murine ovarian cells in order to explain the change in mRNA expression pattern [37]. Ovarian function repair via MenSCs was analyzed in a study with mouse models. Following transplantation, a higher number of live births was observed after mating which supports the idea of MenSCs having a beneficial effect on reproductive function. Researchers discovered this was because of improved homeostasis within the ovarian microenvironment through MenSC regulation of the ECM-dependent FAK/AKT signaling pathway. This is a point to note because the extracellular matrix (ECM) plays a role in cell signaling along with providing structural support to cells. The FAK/AKT pathway is crucial for the upkeep of ovarian function. Because MenSCs were found to regulate this pathway, reproductive and microenvironmental outcomes are enhanced [38]. One final point that solidifies MenSC effectiveness for ovarian function is a human clinical trial that involved fifteen with poor ovarian response. Hormonal treatment was not helping to encourage an ovarian response so researchers injected these women with MenSCs derived from their own menstrual blood. As a result, the number of women who became pregnant and had live births increased significantly compared to the control group who had no treatment This indicates a promising therapeutic option for poor ovarian responses with MenSCs [39] (Figures 14 and 15).
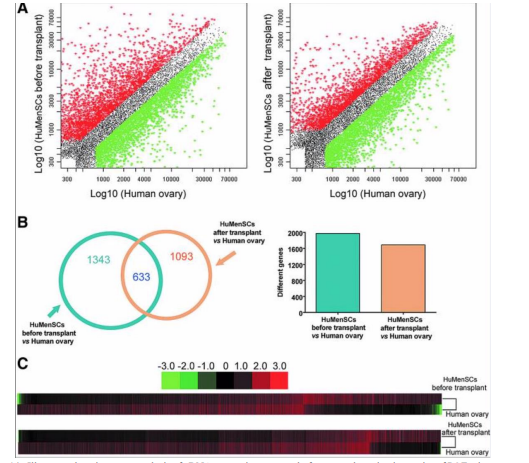
Figure 14: Illustrates the microarray analysis of cDNA expression patterns before transplantation in ovaries of POF mice vs normal human ovarian tissue. The scatter plot displays the differing mRNA expression after transplant between MenSCs and normal human ovarian cells [37]
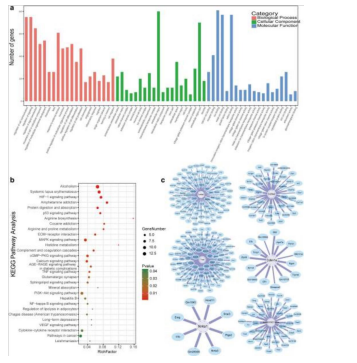
Figure 15: Illustrates the method of “Go enrichment analysis” to understand the anatomy and physiology of certain genes. A KEGG pathway analysis was also used to identify specific pathways between two groups of genes. The analysis of the interaction between these genes was observed via a gene-co-expression network which focused on specific pathways related to ECM-receptor, focal adhesion, and PI3K-AKT signaling [38].
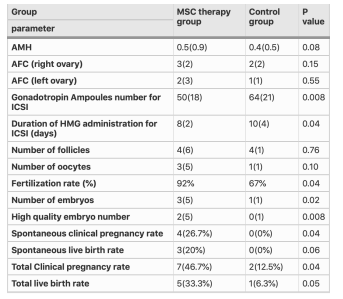
Table 3: Shows a comparison of participant’s characteristics in MenSC therapy and control groups after intervention for poor ovarian response [39].
The therapeutic effects of MenSCs can also be applied to lung injury. In an experimental with mice models, acute lung injury was induced through the exposure of LPS (lipopolysaccharide), a molecule found on the outer membrane. The mice were then injected with MenSCs and observed. The outcome is similar to the trend throughout this discussion of improved function for the various diseases/injuries where inflammation was reduced, anti-inflammatory cytokine secretion successfully combatted high levels of pro-inflammatory cytokines, and the number of infiltrating immune cells in the lungs was suppressed which led to overall improved lung function [40]. Similar results were seen in another study but specifically mentioned the increase of cytokine IL-10. This study also places an emphasis on MenSC environmental interaction that led to improved lung injury score and reduced inflammation markers in the lung fluid, leading to a possible conclusion that MenSC paracrine activity may be more significant than differentiation [41]. Further confirmation of this activity is seen in a study conducted with mice models that had pulmonary fibrosis [PF] where they were observed following MenSC injection. Along with a reduction of inflammation, there was a reduction of collagen deposition and neutrophils, inflammatory cells, were specifically mentioned to be present in lower levels. Though, what is significant is the reduction of collagen as that plays a role in the progression of fibrosis [42]. In conclusion, as far as acute lung injuries and pulmonary fibrosis, MenSCs can help with improved lung function and histology through their paracrine mechanism (Figures 16 and 17).
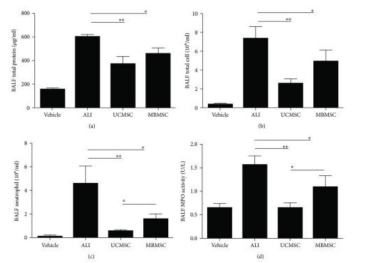
Figure 16: Displays the the identification of the quantity of cells and protein in BALF, which includes [a] the measurement of total protein concentrations, [b] the enumeration of total cell counts, [c] the calculation of neutrophil counts, and [d] the assessment of MPO activity. The experiment involved normal mice with PBS injection as the control group, LPS-induced ALI model as the ALI group, and two transplantation groups-UCMSC and MBMSC - of ALI mice [41]
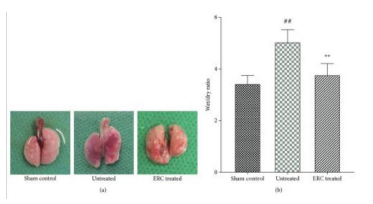
Figure 17: Illustrates ERCs can improving alveolitis and reducing collagen deposition.
A representative figure of gross pathology indicates that the lung in the sham control group was completely smooth, while in the untreated group, it became inflamed, mauve, and had fibrous deposition. After ERC treatment, it showed the opposite of the inflammation and some fibrotic patches. The severity of inflammation was evaluated using the wet/dry ratio, which showed that ERCs reduced the level of wet/dry ratio compared to the untreated group [42].
One final therapeutic effect of MenSCs to discuss is the impact on skin wound repair. One study investigates the effect of these cells the skin of mice that have a persistent wound. Following injection of the MenSCs, the healing process of the wound appeared to be upgraded as wound closure occurred faster and there was better regeneration of the tissue. Angiogenesis appeared to be encouraged by the MenSCs, indicating better blood flow to the site of the wound [43]. Similar research was conducted by another investigator but instead observed the genes at play. Following injection of MenSCs to the wound area, two genes (II-8 and Vegf) that promote the growth of blood vessels were increased, further supporting MenSCs’ effect on angiogenesis. A higher density of collagen fibers was also observed in the group of injected cells, suggesting beneficial reparative properties of MenSCs as collagen is an important component to healthy skin. Improved closure was also noted when the MenSCs were seeded onto an amniotic membrane and applied to the wound area rather than injected [44]. The differentiation potential was investigated in another experiment where MenSCs obtained from healthy, menstruating women were cultured under specific conditions to induce specialization into keratinocyte-like cells. The results showed successful differentiation into cells that were similar to human keratinocytes and the specialized MenSCs cells expressed markers specific to keratinocytes, such as involucrin and cytokeratin 14, which are involved in the epidermal barrier formation [45]. This can be seen on a 3D culture of a skin wound to observe the wound healing process where effects were more enhanced compared to a 2D culture, suggesting ways to culture differentiated MenSCs more efficiently [46]. The same analysis was conducted for biomimetic nanofibrous scaffolding where researchers seeded MenSCs on a nanofibrous scaffold that micmicked the ECM of the skin versus a regular tissue culture plate. Findings showed an improved healing capacity along with expression of genes involved in tissue regeneration and wound healing such as transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) [47]. There was also increased migration and proliferation and improved keratinocyte differentiation, just like the 3D model; thus, highlighting two methods for improved wound healing capacity (Figures 18 and 19).
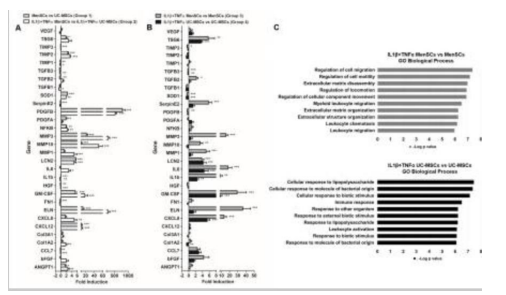
Figure 18: Illustrates a differential transcription pattern of genes associated with wound healing as seen in MenSCs [45].
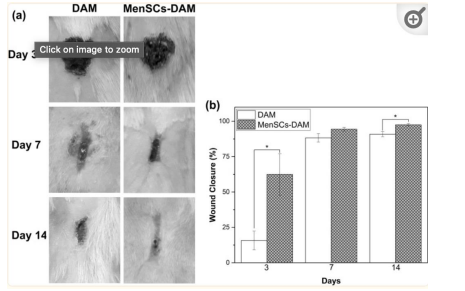
Figure 19: Illustrates macroscopic appearances of skin wounds treated with DAM and MenSCs 3, 7, and 14 days post wounding along with a histogram comparing the wound closure [46]
Conclusion
The field of menstrual-derived stem cells is still an ever growing field with much potential to change the way we view the treatment of disease and perhaps medicine in general. They are easily obtained, pose no ethical conflicts, affordable, and unlimited with its monthly regenerative capacity. MenSCs contain a unique set of characteristics that define it over the other stem cells, but its two most important properties are its differentiation and paracrine activity. These properties play a critical role in the therapeutic effects of cardiac health, neurological disease/ damage, liver health, lung health, and skin repair. However, despite its benefits, the field of MenSCs have limited capabilities. Because almost all the studies around MenSCs present clinical investigations in mice/rats, there is limited relevance to humans. Larger animal models must be utilized in order to address this issue and have a better understanding of the effects of MenSCs before using them on humans more. It has also been found that only a small number of infused MSCs remain in the body after administration and few appear to integrate into tissues. This is because the environment of diseased tissue/organ can hinder MSC integration. However, MSCs can still have positive effects through their paracrine actions, which reset the immune system and promote cellular repair without substantial integration [18]. Despite having an unlimited supply, there is little understanding in the attitudes/willingness of women to donate their menstrual blood. In one survey conducted with 100 women [18 years or older], age, medical history, menstrual experience, and willingness to donate/accept MenSCs therapy were assessed. Results indicated no significant relationship amongst the factors surveyed and 78% of menstruating women were willing to donate their menstrual blood. Thus, it is safe to assume that the future of MenSC obtainment is positive for now as women are willing to donate regardless of medical history, blood donation, age, and perception of periods [48]. The therapeutic effect was thoroughly discussed within this paper, but it brings up the question of its true effect on humans. There is also a lack of evidence surrounding long-term effects or sustained therapy leaving the survival of MenSCs in foreign bodies to be unknown [49,50]. Furthermore, Ren et al. discovered that MenSCs are prone to being contaminated by different types of bacteria during the isolation process [41] Women with premature ovarian insufficiency [POI] cannot collect and use their own MenSCs because they no longer have periods [38]. As a result, when considering the ideal amount of MenSCs, the timing of administration, injection methods, and routes for various illnesses, multiple factors must be thoroughly examined. Overall, there is immense potential for MenSC treatment due to its myriad of benefits but further studies are needed to learn more on its mechanisms and impact on humans or a more similar organism.
References
1.Alvarez, C. V., Garcia-Lavandeira, M., Garcia-Rendueles, M. E., Diaz-Rodriguez, E., Garcia-Rendueles, A. R., PerezRomero, S., Vila, T. V., Rodrigues, J. S., Lear, P. V., & Bravo, S. B. (2012). Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. Journal of molecular endocrinology, 49(2), R89–R111.
2. Kolios, G., & Moodley, Y. (2013). Introduction to stem cells and regenerative medicine. Respiration; international review of thoracic diseases, 85(1), 3–10.
3. Zhang, M. J., Liu, B., Xia, W., Sun, Z. Y., & Lu, K. H. (2009). Could cells from menstrual blood be a new source for cell-based therapies?. Medical hypotheses, 72(3), 252–254.
4. Patel, A. N., & Silva, F. (2008). Menstrual blood stromal cells: the potential for regenerative medicine. Regenerative medicine, 3(4), 443–444.
5. Du, H., & Taylor, H. S. (2007). Contribution of bone marrowderived stem cells to endometrium and endometriosis. Stem cells (Dayton, Ohio), 25(8), 2082–2086.
6. Patel, A. N., Park, E., Kuzman, M., Benetti, F., Silva, F. J., & Allickson, J. G. (2008). Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell transplantation, 17(3), 303–311.
7. Sampson, John A. (1940). The Development of the Implantation Theory for the Origin of Peritoneal Endometriosis. American Journal of Obstetrics and Gynecology, 40, 549-557.
8. Patki, S. M., Kadam, S. S., Phadnis, S. M., & Bhonde, R. R. (2008). Who is the culprit for post menopausal syndrome? Uterus/Ovary!. Medical hypotheses, 71(3), 382–385.
9. Gargett C. E. (2007). Uterine stem cells: what is the evidence?. Human reproduction update, 13(1), 87–101.
10. Liu, Y., Niu, R., Yang, F., Yan, Y., Liang, S., Sun, Y., Shen, P., & Lin, J. (2018). Biological characteristics of human menstrual blood-derived endometrial stem cells. Journal of cellular and molecular medicine, 22(3), 1627–1639.
11. Gargett, C. E., Schwab, K. E., & Deane, J. A. (2016). Endometrial stem/progenitor cells: the first 10 years. Human reproduction update, 22(2), 137–163.
12. Meng, X., Ichim, T. E., Zhong, J., Rogers, A., Yin, Z., Jackson, J., Wang, H., Ge, W., Bogin, V., Chan, K. W., Thébaud, B., & Riordan, N. H. (2007). Endometrial regenerative cells: a novel stem cell population. Journal of translational medicine, 5, 57.
13. Murphy, M. P., Wang, H., Patel, A. N., Kambhampati, S., Angle, N., Chan, K., Marleau, A. M., Pyszniak, A., Carrier, E., Ichim, T. E., & Riordan, N. H. (2008). Allogeneic endometrial regenerative cells: an “Off the shelf solution” for critical limb ischemia?. Journal of translational medicine, 6, 45.
14. Nikoo, S., Ebtekar, M., Jeddi-Tehrani, M., Shervin, A., Bozorgmehr, M., Kazemnejad, S., & Zarnani, A. H. (2012). Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. The journal of obstetrics and gynaecology research, 38(5), 804–809.
15. Luz-Crawford, P., Torres, M. J., Noël, D., Fernandez, A., Toupet, K., Alcayaga-Miranda, F., Tejedor, G., Jorgensen, C., Illanes, S. E., Figueroa, F. E., Djouad, F., & Khoury, M. (2016). The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem cells (Dayton, Ohio), 34(2), 456–469.
16. Shokri, MR., Bozorgmehr, M., Ghanavatinejad, A. et al. (2019). Human menstrual blood-derived stromal/stem cells modulate functional features of natural killer cells. Sci Rep 9, 10007.
17. Alcayaga-Miranda, F., Cuenca, J., Martin, A., Contreras, L., Figueroa, F. E., & Khoury, M. (2015). Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem cell research & therapy, 6, 199.
18. Bozorgmehr, M., Gurung, S., Darzi, S., Nikoo, S., Kazemnejad, S., Zarnani, A. H., & Gargett, C. E. (2020). Endometrial and Menstrual Blood Mesenchymal Stem/ Stromal Cells: Biological Properties and Clinical Application. Frontiers in cell and developmental biology, 8, 497.
19. Hida, N., Nishiyama, N., Miyoshi, S., Kira, S., Segawa, K., Uyama, T., Mori, T., Miyado, K., Ikegami, Y., Cui, C., Kiyono, T., Kyo, S., Shimizu, T., Okano, T., Sakamoto, M., Ogawa, S., & Umezawa, A. (2008). Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem cells (Dayton, Ohio), 26(7), 1695–1704.
20. Jiang, Z., Hu, X., Yu, H., Xu, Y., Wang, L., Chen, H., Chen, H., Wu, R., Zhang, Z., Xiang, C., Webster, K. A., & Wang, J. A. (2013). Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. Journal of cellular and molecular medicine, 17(10), 1247–1260.
21. Cui, C. H., Uyama, T., Miyado, K., Terai, M., Kyo, S., Kiyono, T., & Umezawa, A. (2007). Menstrual bloodderived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Molecular biology of the cell, 18(5), 1586–1594.
22. Jiang, Z., Hu, X., Yu, H., Xu, Y., Wang, L., Chen, H., Chen, H., Wu, R., Zhang, Z., Xiang, C., Webster, K. A., & Wang, J. A. (2013). Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. Journal of cellular and molecular medicine, 17(10), 1247–1260.
23. Xu, X., Li, X., Gu, X., Zhang, B., Tian, W., Han, H., Sun, P., Du, C., & Wang, H. (2017). Prolongation of Cardiac Allograft Survival by Endometrial Regenerative Cells: Focusing on B-Cell Responses. Stem cells translational medicine, 6(3), 778–787.
24. Liu, Y., Niu, R., Li, W., Lin, J., Stamm, C., Steinhoff, G., & Ma, N. (2019). Therapeutic potential of menstrual bloodderived endometrial stem cells in cardiac diseases. Cellular and molecular life sciences : CMLS, 76(9), 1681–1695.
25. Borlongan, C. V., Kaneko, Y., Maki, M., Yu, S. J., Ali, M., Allickson, J. G., Sanberg, C. D., Kuzmin-Nichols, N., & Sanberg, P. R. (2010). Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem cells and development, 19(4), 439–452.
26. Kaneko, Y., Dailey, T., Weinbren, N. L., Rizzi, J., Tamboli, C., Allickson, J. G., Kuzmin-Nichols, N., Sanberg, P. R., Eve, D. J., Tajiri, N., & Borlongan, C. V. (2013). The battle of the sexes for stroke therapy: female- versus male-derived stem cells. CNS & neurological disorders drug targets, 12(3), 405–412.
27. Zhao, Y., Chen, X., Wu, Y., Wang, Y., Li, Y., & Xiang, C. (2018). Transplantation of Human Menstrual BloodDerived Mesenchymal Stem Cells Alleviates Alzheimer’s Disease-Like Pathology in APP/PS1 Transgenic Mice.Frontiers in molecular neuroscience, 11, 140.
28. Wolff, E. F., Gao, X. B., Yao, K. V., Andrews, Z. B., Du, H., Elsworth, J. D., & Taylor, H. S. (2011). Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. Journal of cellular and molecular medicine, 15(4), 747–755.
29. Farzamfar, S., Naseri-Nosar, M., Ghanavatinejad, A., Vaez, A., Zarnani, A. H., & Salehi, M. (2017). Sciatic nerve regeneration by transplantation of menstrual blood-derived stem cells. Molecular biology reports, 44(5), 407–412.
30. Ruff, C. A., & Fehlings, M. G. (2010). Neural stem cells in regenerative medicine: bridging the gap. Panminerva medica, 52(2), 125–147.
31. Wu, Q., Wang, Q., Li, Z., Li, X., Zang, J., Wang, Z., Xu, C., Gong, Y., Cheng, J., Li, H., Shen, G., & Dong, C. (2018). Human menstrual blood-derived stem cells promote functional recovery in a rat spinal cord hemisection model. Cell death & disease, 9(9), 882.
32. Yan, Y., Wang, X., & Zhu, G. (2022). Endometrium Derived Stem Cells as Potential Candidates in Nervous System Repair. Annals of biomedical engineering, 50(5), 485–498.
33. Khanjani, S., Khanmohammadi, M., Zarnani, A. H., Talebi, S., Edalatkhah, H., Eghtesad, S., Nikokar, I., & Kazemnejad, S. (2015). Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. Journal of tissue engineering and regenerative medicine, 9(11), E124– E134.
34. Fathi-Kazerooni, M., Tavoosidana, G., Taghizadeh-Jahed, M., Khanjani, S., Golshahi, H., Gargett, C. E., Edalatkhah, H., & Kazemnejad, S. (2017). Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy, 19(12), 1474–1490.
35. Chen, L., Zhang, C., Chen, L., Wang, X., Xiang, B., Wu, X., Guo, Y., Mou, X., Yuan, L., Chen, B., Wang, J., & Xiang, C. (2017). Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem cells translational medicine, 6(1), 272–284.
36. Manshadi, M. D., Navid, S., Hoshino, Y., Daneshi, E., Noory, P., & Abbasi, M. (2019). The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microscopy research and technique, 82(6), 635–642.
37. Liu, T., Huang, Y., Zhang, J., Qin, W., Chi, H., Chen, J., Yu, Z., & Chen, C. (2014). Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem cells and development, 23(13), 1548–1557.
38. Feng, P., Li, P., & Tan, J. (2019). Human Menstrual BloodDerived Stromal Cells Promote Recovery of Premature Ovarian Insufficiency Via Regulating the ECM-Dependent FAK/AKT Signaling. Stem cell reviews and reports, 15(2), 241–255.
39. Zafardoust, S., Kazemnejad, S., Darzi, M., Fathi-Kazerooni, M., Rastegari, H., & Mohammadzadeh, A. (2020). Improvement of Pregnancy Rate and Live Birth Rate in Poor Ovarian Responders by Intraovarian Administration of Autologous Menstrual Blood Derived- Mesenchymal Stromal Cells: Phase I/II Clinical Trial. Stem cell reviews and reports, 16(4), 755–763.
40. Xiang, B., Chen, L., Wang, X., Zhao, Y., Wang, Y., & Xiang, C. (2017). Transplantation of Menstrual BloodDerived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. International journal of molecular sciences, 18(4), 689.
41. Ren, H., Zhang, Q., Wang, J., & Pan, R. (2018). Comparative Effects of Umbilical Cord- and Menstrual Blood-Derived MSCs in Repairing Acute Lung Injury. Stem cells international, 2018, 7873625.
42. Zhao, Y., Lan, X., Wang, Y., Xu, X., Lu, S., Li, X., Zhang, B., Shi, G., Gu, X., Du, C., & Wang, H. (2018). Human Endometrial Regenerative Cells Attenuate BleomycinInduced Pulmonary Fibrosis in Mice. Stem cells international, 2018, 3475137.
43. Cuenca, J., Le-Gatt, A., Castillo, V., Belletti, J., Díaz, M., Kurte G, M., Gonzalez, P. L., Alcayaga-Miranda, F., Schuh, C. M. A. P., Ezquer, F., Ezquer, M., & Khoury, M. (2018). The Reparative Abilities of Menstrual Stem Cells Modulate the Wound Matrix Signals and Improve Cutaneous Regeneration. Frontiers in physiology, 9, 464.
44. Farzamfar, S., Salehi, M., Ehterami, A., Naseri-Nosar, M., Vaez, A., Zarnani, A. H., Sahrapeyma, H., Shokri, M. R., & Aleahmad, M. (2018). Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomedical engineering letters, 8(4), 393–398.
45. Akhavan-Tavakoli, M., Fard, M., Khanjani, S., Zare, S., Edalatkhah, H., Mehrabani, D., Zarnani, A. H., Shirazi, R., & Kazemnejad, S. (2017). In vitro differentiation of menstrual blood stem cells into keratinocytes: A potential approach for management of wound healing. Biologicals : journal of the International Association of Biological Standardization, 48, 66–73.
46. Fard, M., Akhavan-Tavakoli, M., Khanjani, S., Zare, S., Edalatkhah, H., Arasteh, S., Mehrabani, D., Zarnani, A. H., Kazemnejad, S., & Shirazi, R. (2018). Bilayer Amniotic Membrane/Nano-fibrous Fibroin Scaffold Promotes Differentiation Capability of Menstrual Blood Stem Cells into Keratinocyte-Like Cells. Molecular biotechnology, 60(2), 100–110.
47. Arasteh, S., Katebifar, S., Shirazi, R., & Kazemnejad, S. (2020). Differentiation of Menstrual Blood Stem Cells into Keratinocyte-Like Cells on Bilayer Nanofibrous Scaffold. Methods in molecular biology (Clifton, N.J.), 2125, 129– 156.
48. Manley, H., Sprinks, J., & Breedon, P. (2019). Menstrual Blood-Derived Mesenchymal Stem Cells: Women’s Attitudes, Willingness, and Barriers to Donation of Menstrual Blood. Journal of women’s health (2002), 28(12), 1688–1697.
49. Chen, L., Qu, J., Cheng, T., Chen, X., & Xiang, C. (2019). Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem cell research & therapy, 10(1), 406.
50. Bianco, P., Cao, X., Frenette, P. S., Mao, J. J., Robey, P. G., Simmons, P. J., & Wang, C. Y. (2013). The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature medicine, 19(1), 35–42
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



