Case Report - (2023) Volume 8, Issue 4
Successful management of late leakage after sleeve gastrectomy: A case of a challenging delayed complication
Received Date: Mar 28, 2023 / Accepted Date: Apr 03, 2023 / Published Date: Apr 15, 2023
Abstract
We report a unique case of delayed complication of a 36-year-old obese female patient who had a very late gastric leak, namely, four years after LSG. Patient presented with worsening abdominal pain and discomfort, anemia, intermittent fever of 39°C, vomiting and a general feeling of illness and anxiety. Clinical, laboratory and instrumental studies (X-Ray, CT, US) revealed the leakage from the gastric remnant at the gastroesophageal junction level and a small left subdiaphragmatic collection. Revisional laparoscopy and intraoperative methylene blue test (MBT) did not reveal a defect and leakage of the suture line. On rehospitalization the surgical intervention was recommended. The successful extirpation of the stomach remnant with resection of distal segment of esophagus and Roux-en-Y reconstruction was performed. The control X-Ray examination was performed at 6th p/o day, which reveals no extravasation and free passage of contrast to the distal part of GI tract. The patient started peroral eating and was discharged the Hospital at 10th p/o day. At control X-Ray examination after 1 month of surgery the patient is doing well. We consider, that this surgical procedure seems to be the most optimal and radical surgical intervention in similar cases.
Keywords
Laparoscopic Sleeve Gastrectomy, Late Leakage, Roux-en-Y reconstruction
Introduction
Recently the laparoscopic sleeve gastrectomy (LSG) has become one of the most requested and frequently performed bariatric surgical procedures for obesity management worldwide [1-18]. At the present time more than half of all the bariatric surgeries accounted for gastric sleeve surgeries, according to the latest IFSO reports. The post-laparoscopic gastric sleeve leak is the most serious and feared complication of this procedure as it is associated with significant morbidity and mortality [1]. By definition a gastrointestinal anastomotic leak is “a leak of luminal contents from a surgical join between two hollow viscera”, according to a standard definition provided by the UK Surgical Infection Study Group [3]. A leak can be classified according to the time of their emergence as [2,9,13]:
Early leak:Which may appear between day 1 and 3 after surgery Intermediate leak:Which appears between day 4 and 7 after surgery Late leak: It develops during or after day 8 of surgery Herein, we report and discuss a unique case of delayed complication of a 36-year-old obese female patient who had a very late gastric leak, namely, four years after LSG.
Case Presentation
A 36-year-old female patient presented with worsening abdominal pain and discomfort, anemia, intermittent fever of 39°C, vomiting and a general feeling of illness and anxiety. From her medical history it became known that 4 years ago she underwent standard LSG bariatric procedure with an estimated body mass index (BMI) of 49 kg/m2. Performed clinical, laboratory and instrumental studies (X-Ray, CT US) revealed the following clinical picture: leakage from the gastric remnant at the gastroesophageal junction level and a small left subdiaphragmatic collection (Figure 1). Revisional laparoscopy was performed without significant intraoperative findings of any leak and as intraoperative methylene blue test (MBT) was negative the prophylactic drainage tube was placed at the left subdiaphragmatic space. The patient was discharged the Hospital after 1 week with clinical improvement, but came back after 2 weeks in a worse condition. Taking into account the existing clinical picture and the possibility of developing severe complications the surgical intervention was recommended to avoid life threatening complications.
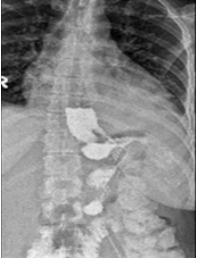
Figure 1: Preoperative X-Ray Data.
Operative Treatment
Upper midline laparotomy and expose of abdominal cavity was performed. The pronounced adhesive process in the upper part of abdominal cavity was revealed. After the sharp and blunt dissection with technical difficulties mobilization of stomach remnant and abdominal segment of esophagus was performed. The longitudinal defect with necrotic edges up to 1cm long at the left posterior side near the EG junction and abscess at left subdiaphragmatic pace was revealed (Figure 2). Due to the pronounced necrotic and infiltration changes in the stomach wall and esophagus the decision to perform extirpation of the stomach remnant with resection of distal segment of esophagus was made (Figure 3). Roux-en-Y esophago-jejunostomy and jejunojejunostomy anastomoses with using the surgical staplers were performed for the reconstruction of GI tract integrity. Sanitation and drainage of the subdiaphragmatic abscess completed the operation. Multimodal anesthesia approach was used for this major surgical procedure, which took almost 6 hours.
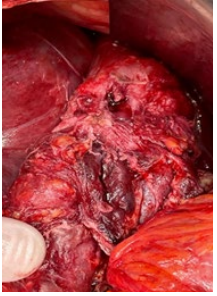
Figure 2: Mobilized abdominal esophagus with defect.
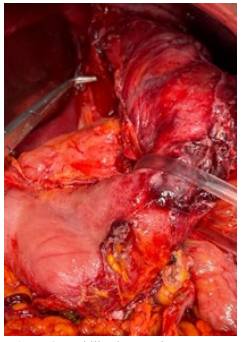
Figure 3: Mobilized stomach remnant.
Results
Postoperative course was uneventful and without any serious complications. The control X-Ray examination was performed at 6th p/o day, which reveals no extravasation and free passage of contrast to the distal part of GI tract. Only in the postoperative period the patient required a puncture of the left pleural cavity to evacuate pleural exudate. The patient started peroral eating and was discharged the Hospital at 10th p/o day. At control X-Ray examination after 1 month of surgery the patient is doing well, eats often small portions of semi-liquid food and does not show any special complaints (Figure 4).
<img src="https://www.opastpublishers.com/scholarly-images/5356-67aad84399dcf-successful-management-of-late-leakage-after-sleeve-gastrecto.png" style="width:300px;height:200px;">
Figure 4: Postoperative X-Ray Data.
Discussion
LSG was first performed in 2000, by M.Gagner and E.Patterson, as part of a duodenal switch procedure [6]. Since that time LSG has become popular and the most commonly performed bariatric surgical procedure due to its simplicity and low complication rate. Currently, many surgeons are considering and performing LSG as a stand-alone procedure as effective weight loss option for the obese patient. As a result, at the present time the LSG became the most requested and practiced bariatric surgery worldwide. However, as every medical treatment and surgery comes with its own risks the at first glance, a simple and technically easy LSG is also not risk-free procedure, with the most serious risk being the staple line leak and bleeding. Staple line leak is the most severe, dangerous and possibly lifethreatening complication of LSG as it is difficult to treat and is associated with significant morbidity and even mortality. The incidence and leak rate reported on bariatric literature varies between 1 to 5% of patients that undergo primary gastric sleeve surgery and 16-20% for reoperative surgery where a previous gastric operation has been performed [1-5,8-12]. Some authors have been proposed that according to the severity gastric leaks have been classified as follows:
1. Type I or subclinical: This corresponds to local leakage, with no spillage or dissemination, through a fistulous track to the pleural or abdominal cavity or the appearance of contrast material in any abdominal drain.
2. Type II or clinical: This corresponds to leakage with great dissemination or diffusion to the pleural or abdominal cavity, by way of an irregular pathway, with appearance of contrast medium in any of the abdominal drains [2,9,13].
The management and intervention options for a leak after a gastric sleeve surgery varies according to the clinical situation, the time of diagnosis and the location of the leak [3]. According to the First International Consensus Summit for Sleeve Gastrectomy, treatment of leak included early oversewing, drainage (CAT or open), endoscopic clipping, and persisting fistula requiring fibrin glue, stents, Roux-loop, and even total gastrectomy. There is no consensus according the standard treatment algorithm and so, the strategy and treatment plan for dealing with leaks should be individualized based on a clinical presentation (4,7,8-11,16-18). Remembering the old surgical aphorism, that “prophylaxis is the best treatment” G. Loo et al., (2019) have proposed several methods that may decrease stapleline leaks in LSG. These include the use of a bougie size greater than 40 Fr, routine use of methylene blue test during surgery, beginning transection at 2-6 cm from the pylorus, mobilizing the fundus before transection, and staying away from the GOJ at the last firing. Other methods include the proper alignment of the staple-line, control of staple-line bleeding, and performing staple-line reinforcement. It should be noted that even a negative intraoperative MBT does not eliminate the possibility of a leak, which can lead to the false path of expectant tactics of conservative treatment. However, the failure of conservative treatment will any way dictate the need for more radical and aggressive surgical treatment. And our case, which we have described above, fully confirms this assumption.
Conclusion
The management of a staple-line leak still remains challenging condition, which could be the life-threatening complication with fatal outcome. It is also difficult to diagnose the late leakage because its symptoms may vary and because some radiological studies and test might give false results, which lead to choose the wrong treatment tactics. Our case illustrates, that staple-line leak could be revealed after the years of primary LSG procedure which could lead to serious and challenging problems. Every bariatric patient should be under close outpatient monitoring for their follow-up consultations and tests, even if they are feeling well. Early diagnosis of the leak is fundamental for determination of the most optimal immediate treatment method to avoid life threatening complications. Along with other methods of treatment, it is necessary to take into account in some cases the possibility and necessity of performing the extirpation of the stomach remnant with subsequent Roux-en-Y reconstruction.
References
1. Mohamed, A. A., Humaida, A. A., & Qureshi, A. S. (2021). Delayed Post-Laparoscopic Sleeve Gastrectomy Leak Successfully Treated With Endoscopic Clips and Tissue Adhesive: Case Report and Literature Review. Cureus, 13(4), e14532.
2. Burgos, A. M., Braghetto, I., Csendes, A., Maluenda, F., Korn, O., Yarmuch, J., & Gutierrez, L. (2009). Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obesity surgery, 19(12), 1672–1677.
3. Abou Rached, A., Basile, M., & El Masri, H. (2014). Gastric leaks post sleeve gastrectomy: review of its prevention and management. World journal of gastroenterology, 20(38), 13904-13910.
4. Zanotti, D., Elkalaawy, M., Mohammadi, B., Hashemi, M., Jenkinson, A., & Adamo, M. (2015). Gastro-cutaneous fistula 4 years after a fully resolved staple line leak in sleeve gastrectomy. Journal of surgical case reports, 2015(12), rjv152.
5. Sakran, N., Goitein, D., Raziel, A., Keidar, A., Beglaibter, N., Grinbaum, R., Matter, I., Alfici, R., Mahajna, A., Waksman, I., Shimonov, M., & Assalia, A. (2013). Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surgical endoscopy, 27(1), 240-245.
6. Gagner, M., &Patterson, E. (2000) Laparoscopic biliopancreatic diversion with duodenal switch. Digestive Surgery, 17, 547-566.
7. Deitel, M., Crosby, R. D., & Gagner, M. (2008). The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25-27, 2007. Obesity surgery, 18(5), 487-496.
8. Dakwar, A., Assalia, A., Khamaysi, I., Kluger, Y., & Mahajna, A. (2013). Late complication of laparoscopic sleeve gastrectomy. Case reports in gastrointestinal medicine, 2013, 136153.
9. Csendes, A., Braghetto, I., León, P., & Burgos, A. M. (2010). Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract, 14(9), 1343-1348..
10. Abosonna, K.A., El-Hady, H.A., & Yousef, H.A. et al. (2022) Leakage Post Laparoscopic Sleeve Gastrectomy. AIMJ, 3, 57-62.
11. Nocca, D., Krawczykowsky, D., Bomans, B., Noël, P., Picot, M. C., Blanc, P. M., de Seguin de Hons, C., Millat, B., Gagner, M., Monnier, L., & Fabre, J. M. (2008). A prospective multicenter study of 163 sleeve gastrectomies: results at 1 and 2 years. Obesity surgery, 18(5), 560-565.
12. Himpens, J., Dapri, G., & Cadière, G. B. (2006). A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obesity surgery, 16(11), 1450- 1456.
13. Praveenraj, P., Gomes, R. M., Kumar, S., Senthilnathan, P., Parthasarathi, R., Rajapandian, S., & Palanivelu, C. (2016). Management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity: A tertiary care experience and design of a management algorithm. Journal of minimal access surgery, 12(4), 342-349.
14. Bashah, M., Khidir, N., & El-Matbouly, M. (2020). Management of leak after sleeve gastrectomy: outcomes of 73 cases, treatment algorithm and predictors of resolution. Obesity surgery, 30(2),
15. Loo, G. H., Rajan, R., & Nik Mahmood, N. R. K. (2019). Staple-line leak post primary sleeve gastrectomy. A two patient case series and literature review. Annals of medicine and surgery (2012), 44, 72-76.
16. Casella, G., Soricelli, E., Rizzello, M., Trentino, P., Fiocca, F., Fantini, A., Salvatori, F. M., & Basso, N. (2009). Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obesity surgery, 19(7), 821-826.
17. Praveenraj, P., Gomes, R. M., Kumar, S., Senthilnathan, P., Parthasarathi, R., Rajapandian, S., & Palanivelu, C. (2016). Management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity: A tertiary care experience and design of a management algorithm. Journal of minimal access surgery, 12(4), 342-349.
18. Tan, J. T., Kariyawasam, S., Wijeratne, T., & Chandraratna, H. S. (2010). Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obesity surgery, 20(4), 403-409.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



