Case Report - (2023) Volume 8, Issue 6
Sister Mary Joseph Nodule, Umbilical Sentinel for an Endometrioid Endometrial Carcinoma in Postmenopause-Challenges for a Multidisciplinary Approach
2”Carol Davila” University of Medicine and Pharmacy, Romania
3“Dr Ioan Cantacuzino” Laboratory of Pathology, Bucharest, Romania
Received Date: May 16, 2023 / Accepted Date: May 22, 2023 / Published Date: Jun 01, 2023
Abstract
A 82 years old, hypertensive, obese, 1 delivery, menopause at 47 yrs, non-smoker, with history of vaginal bleeding in January 2018, is sent by the dermatologist for an irregular umbilical tumor of 2/1 cm, recurrent at 6 weeks post-ablation, microscopically considered as a carcinomatous metastasis. Abdomino-pelvic MRI rises the suspicion of uterine carcinoma, which is confirmed by endometrial biopsy. It is done extrafascial total hysterectomy with bilateral salpingo-oophorectomy, and pelvic lymph dissection and ablation, plus ablation of a skin-adipose-conjunctive peri-and subumbilical area of 5/4 cm. There were no peritoneal metastases, or other viscera abnormalities. The optic microscopy shows moderate differentiated endometrioid endometrial carcinoma, with areas of squamous differentiation, invasion in external myometrial half, without peritoneal invasion, with vessels metastatic embolism, positive pelvic lymph nodes with moderate differentiated endometrioid endometrial carcinoma and desmoplastic reaction; left ovary with mature teratoma. Umbilical specimen has malignant invasion with cribriform and tubular pattern, areas of squamous differentiation, and malignant emboli in vessels. The patient suffered radiotherapy, under oncologic monitoring. The follow up at 6,12,18 and 36 months was with no recurrence in genital, pelvis, peritoneum, abdominal wall. Sister Mary Joseph Nodule (SMJN) has an old history: first observations of assistant catholic nun Mary Joseph Dempsey (Saint Mary’s Hospital, Rochester, Minnesota, USA), William Mayo (1928) description, Hamilton Bailley (1949) characterization. Literature associates SMJN to cancers originating in gastro-intestinal, colonic tract, respiratory, urinary and genital tract: primary ovarian and endometrial cancers; Romanian case is the 36th with endometrial origin. Umbilical invasion may be due to direct vessels’ embolization by malignant endometrial cells, via lymphatics which run along the obliterated umbilical vein, or via the remnant structures of the falciform and umbilical ligaments. Patient is under oncologic monitoring on hormone therapy (Megesin®), with good prognosis up to four years of follow up, different from literature mentioned bad prognosis
Keywords
Cutaneous metastases, Sister mary joseph nodule, Endometrioid endometrial carcinoma
Introduction
Endometrial carcinoma has an increasing rate during the last 15- 20 years, all over the world, mainly in USA, as the longitudinal multicentric, multi-ethnic population Study of Women’s Health Across the Nation shows an increase of endometrial cancer of 2.5% during 1992 to 2002, and after both arms of Women Health Initiative closure in 2002/2003, one registered an annually increase with 10% from 2006 to 2012 (trend test 0.82), in women of 50-74 years of age, but also in perimenopause and younger women, below 30 years, presumably connected to obesity, diabetes mellitus [1]. One knows that many endometrial carcinoma may progress without postmenopausal hemorrhage according to postmenopausal cervical stenosis, being discovered at autopsy of old women’s death from other causes. Skin and nails abnormalities are known since long time as sentinel for apparent silent, advanced malignancies-paraneoplastic dermatoses which are discussed in many papers, being a systematic review for gynecological origins [2], or for other severe diseases [3], inducing the ultrasound characteristics-“tip of an iceberg” for the Polish researchers [4]. Umbilicus area may be site for hernia, which may hide sometimes a cancer [5], benign and malignant tumors, and an unusual metastatic site in advanced cancers [6] with primary location in digestive (stomach, colon, pancreas, gall blader), respiratory, urinary and genital (endometrium, endometriosis, cervix, ovaries, fallopian tubes) tracts, with approximately 15-30% of primary tumor remaining occult [7]. The literature presents umbilical skin metastastic tumor nominated “Sister Mary Joseph Nodule” (SMJN) after the name of the catholic sister Mary Joseph Dempsey, who first observed it at Saint Mary’s Hospital -actually Mayo Clinic, in Rochester, Minnesota (USA). The umbilical nodules have wide range of presentation from small (<2 cm) to large as 10 cm, sometimes painful, ulcerated mass with sero-sanguineous to bloody discharge [8], coloring the lingerie, or one may discover a diffuse subcutaneous induration, which can be associated to peritoneal carcinomatosis, which worsens the outcome [9]. The Romanian case highlights the assessment challenges when one registers an umbilical nodule, an external manifestation, that must be considered sentinel for an underlying malignancy or other serious disease.
Case Report
A 82 years old, hypertensive, obese, one spontaneous vaginal delivery, menopause at 47 years, non-smoker, non alcohol drinker, with history of vaginal bleeding some weeks before medical presentation, is sent by dermatologist in January 2018 for gynecological examination for an irregular, firm-elastic umbilical tumor of 2/1 cm, with bumpy, irregular surface, without subjective symptoms, which reappeared at 6 weeks post dermatologist’s ablation. The microscopy appreciated it as a carcinomatous metastasis with no precisely primary organ, and dermatologist presumed genital area- uterus or ovaries, in accordance to the episode of vaginal hemorrhage in late postmenopause, from 47 years of age. At the moment of first gynecological examination, the patient presents moderate anemia, high level of human epididymis protein 4 (HE4), recognized as marker for serous ovarian malignant tumors, but also in cases with high uric acid levels, as patient had; normal EKG, chest X ray in age limits. Ultrasound assessment shows an ovarian mass suggestive for teratoma, enlarged uterus, and endometrium>6 mm thickness. Abdomen-pelvic MRI reveals normal uterine shape and contour, endometrial enlargement of 6.8 mm from fundus to isthmus, an ovarian cyst of 7/4.5 cm suggestive for dermoid cyst, left-sided lymphadenopathy of external iliac nodes, and no metastases in abdominal and pelvic cavities. Histological testing of Pipelle endometrial biopsy is suggestive for medium and weak differentiated endometroioid carcinoma. It is done abdominal laparatomy, with discovery of low amount of peritoneal fluid- cytology negative, enlarged uterus for patient’s age, left ovary with a 2/2 cm cyst, no peritoneal or omental metastasis, or other viscera pathological changes. It is done extrafascial total hysterectomy with bilateral salpingo-oophorectomy, and pelvic lymph nodes dissection and ablation, plus excision of a skin-adipose-conjunctive peri and subumbilical area of 5/4 cm, centered by umbilicus with it’s skin tumor. The optic microscopy shows uterine walls with moderate and weak differentiated endometrioid endometrial carcinoma, with dermoplastic reaction, and compact areas of squamous differentiation, and small pseudoglandular structures, with secondary branches in a reduced stromal mass, invasion of external half of myometrial walls, without peritoneal invasion, vessels with metastatic emboli (Figure 1A,B). The tumor invasion is extended to isthmus and endocervix. Pelvic lymph nodes have invasion with moderate differentiated endometrioid endometrial carcinoma and desmoplastic reaction. Left ovary has a mature teratoma- dermoid cyst. The skin of umbilical specimen has an exophytic tumor, and malignant invasion of cribriform and tubular pattern, areas of squamous differentiation like the uterus, vessels with malignant emboli. Figure 2 presents the emboli from uterine tumor (A) and umbilical skin-adipose conjunctive area, centered by metastatic tumor (B). Table 1 presents the results of immunehistochemistry for the uterus and peri-and sub-umbilical skin-adipose conjunctive tissue.
| Uterus/Endometrium | Umbilical Area |
|---|---|
| ER positive in 90% of tumor cells | P40 positive in epidermis |
| PGR diffuse positive in the proliferative tumor of uterine wall and cervix stroma | Ki67 positive in near 70% of tumor cells |
| Ki67 positive in near 65% of tumor cells | ER positive in 95% of tumor cells |
| WT1 negative in tumor, and zonal positive in stroma | PGR in 90% of tumor cells |
| Legend: ER: estrogen receptors; PGR: progesterone receptors; WT1: vimentin; P40: protein 40 | |
Table 1: Immunehistochemistry of primary uterine/endometrium carcinoma and SMJN.

Figure 1: A) Moderate and weak differentiated endometrioidendometrial adenocarcinoma; B) Compact areas with squamous differentiation (Hematoxilin-eosine (HE) stain x 100; “Dr. I. Cantacuzino” Laboratory of Pathology).
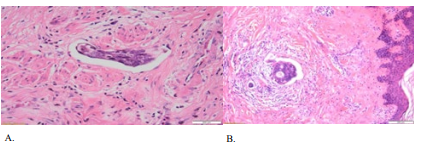
Figure 2: A) Vascular Thrombus in the uterine fragment (HE stain x 40); B) Malignant Embolus in umbilical dermis (HE stain x 20) (“Dr. I. Cantacuzino” Laboratory of Pathology).
Patient had a normal postsurgery evolution, per primam skin healing, being under low molecular weight heparins for 4 weeks postsurgery, for thromboembolism prophylaxis, and discharged at 7 days after surgery. After the pathological results, one decided radiotherapy 45Gy/25 fractions plus brachitherapy 20gy, and Megesin®160mg/day from January 2019, under oncologic follow-up. One registers normal abdominal wall appearance in March 2019 (first year after surgery), Figure 3.
The patient was under oncologic monitoring at every 6 months: clinical, cytological with Bethesda smear and, computerized tomography (CT) annualy. In July 2022 at last visit of follow up (approximately 48 months postsurgery), there were no signs of umbilical, vaginal reccurence or other metastases, clinically and imagistically depicted (CT of thorax, abdomen, pelvis); patient was with the same weight, good spirit, with signs of cardiac dysfunction: both legs’ edema, atrial fibrilation under drugs control, as it was her blood pressure; recurrent genital candidiosis. She was sent to cardiologist to exclude the Trousseau syndrome, which was described to be a non-bacterial thrombotic endocarditis from uterine cancer in a Japanese old patient [10].
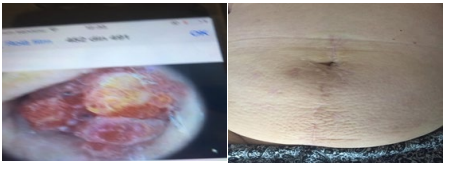
Figure 3: A) Sister Mary Joseph Nodule, 2018, July; B) Abdominal wall and umbilical area appearance, 2019, March.
Discussion
Sister Mary Joseph Nodule Revisited. Many unanswered questions for different medical specialities
Umbilicus is an area of communication with many internal organs through a myriad of vascular and lymphatic pathways [11], and according to these anatomical details umbilicus may be in the center of different pathologies, such as inflammation, infection, or primary neoplastic and metastatic conditions. The dermatologists are confronted to 9 most common paraneoplastic, and metastatic cutaneous manifestations of malignancies in women’s gynecological and breast pathologies [8]: multicentric reticulohistiocytosis, dermatomyositis, malignant acanthosis nigricans, erythema gyratumrepens, hypertrichosis lanuginose acquisita, Sweet syndrome, Paget disease, extramammary Paget disease, and Sister Mary Joseph nodule (SMJN). The metaanalysis of paraneoplastic syndromes [2] occurring from genital tract tumors discusses that it is possible to be discovered in nervous, ophtalmologic, rheumatologic, endocrine, hematologic and renal systems before, at the time, or after the diagnosis of cancer, or at the moment of genital cancers recurrence. SMJN was the alarm symptom/sign in the Romanian case with a moderate differentiated endometrioid endometrial carcinoma with squamous component, for her first presentation to dermatologist, not to gynecology, as it was normal after her first episode of vaginal hemorrhage in postmenopase. The conclusive and accurate diagnosis was difficult, and time consuming, being an endometrial cancer stage IVB (pT2,N1,M1), after FIGO staging (2014) [12] grade I/II histologically, with squamous component, at the age of 82 years, when high grade endometrial cancer is more frequent; obesity, hypertension were the risk factors for this malignancy, which probably progressed during many years from onset, without abnormal vaginal bleeding in postmenopause. The old pathological entity must be revisited. Sir Hamilton Bailley (1949) was the first who characterized the umbilical tumor, at 10 years after Sister Mary Joseph death, in his book “Physical signs in clinical surgery” [13], cited by Powell JL (2011) [14], and after the initial description of William Mayo (1928), who named the entity as “pants button umbilicus”, and after the old observation of superintendent nurse Mary Joseph Dempsey (born Julia Dempsey-1856-1939; before Vatican II, 1965 all the Franciscan nuns had a prefix of Mary as an additional name), a catholic sister at Saint Mary’s Hospital, actual Mayo Clinic, in Rochester, Minnesota (USA) [14]. From the first description of SMJN, one registered many cases of such nodule all over the world, being reported an incidence of 3% [15], in women at ages from 50 to 90 years, and men (with gastric, colonic, genitalia: prostate, testicles [16-19] involvement, without racial or ethnic predominance, both Caucasian and black cases, from Africa (Tanzania), Asia (China, Israel, Japan), Europe (Bosnia and Herzegovina, Bulgaria, Croatia, Germany, Ireland, Romania, UK), North America (Canada, USA), and South America (Brazil). In the Republic of Tanzania retrospective study [20] on 34 patients recorded during 10 years (2003- 2013) it was a ratio of 1.4 to 1 (men to women) (20 (58.8%) men and 14 (41.2%) women), majority with stomach origin (41.1%), adenocarcinoma as more frequent histological type (88.2%), poorly differentiated (52.9%), no uterine/endometrial origin, 2 cases with primary ovarian origin. The South Korea retrospective study (May 2007 to November 2016) analyzed 30 cases with SMJNs (28 from PubMed and 2 from South Korea) [21] and depicted more women (28/30; 93.3%), median age 60.8 years (limits 22 to 89 years), more ovarian primary carcinoma (11/30:36.7%), followed by pancreas (6/30:20.0%), and uterus (6/30:20.0%), colon (3/30;10.0%), testicle (2/30;6.7%), stomach (1/30;3.3%), and vulva (1/30; 3.3%); adenocarcinoma was the most common type (23/30;76.7%), followed by squamous cell carcinoma (4/30;13.3%), adenosquamous carcinoma (1/30;3.3%), mixed tumor (1/30;3.3%), and undifferentiated carcinoma (1/30;3.3%). Generally one discusses that men have metastatic SMJN from primary gastric cancers, and women from primary ovarian cancers. The Romanian case, the endometrial moderate endometrioid carcinoma with squamous component was associated to an ovarian mature teratoma (dermoid cyst), as the Irish published case [6]. Different to these statistical studies of primary origin of an internal tumor signaled by SMJN, there are 15-29% cases [9] with unknown origin of SMJN. One reports at Duke University (USA) during 1988 to 2011, a percentage of 59% from 77 cases of SMJN without the discovery of the primary tumor [22].
Levine D, et al. (2010) [8] in their paper about dermatological diseases signaling other severe pathologies, are discussing an incidence of 28% of all known cases of SMJN to have genital tract primary origin: approximately 34% ovarian cancers, 12% uterine and 5% cervical. Endometriosis was associated to SMJN [23], fallopian tubes (in 2006 being described the forth case of fallopian tube adenocarcinoma signaled by SMJN in a 54 years old Jewish women, who presented for hernia repair) [5]. After the French physicians’ presentation of 368 cases with SMJN from 1960 to 1995 [24] 41.3% being depicted previously to primary malignancy- as in Romanian case, Piura B (2006) [25] physician at Soroka Medical Center, Beer Sheva (Israel) reported a total of 400 published cases of SMJN, and Piura B group (2006) [15] published a total of 27 cases with endometrial carcinomas (low and high grade) depicted after SMJN appearance. The Jewish report is similar to a previous French study (1996) [26] describing 27 cases of SMJN secondary to endometrial carcinoma. After Piura B records (2006) [25], the incidence of endometrial carcinoma signaled by SMJN increased with a low number, as one registered in chronologic order: one endometroid endometrial carcinoma in a Canadian 62 years women (2007) [27]; one endometrial adenocarcinoma in UK (2009) [28];one case in USA (2012) in a 61 years old Afro-American women, SMJN being associated to endometrial poorly differentiated papillary carcinoma, extended to cervix) [29]; one case in Japan (2012) (endometrial squamous cell carcinoma in a 30 years women (2012) [30]; one case in Ireland (2019) [6]; one case in China (2019) (SMJN appeared in a 75 years old women, as a recurrence at 2 years from an endometrial carcinoma, not surgically removed, and treated only by chemotherapy (after 3 cures the sizes of SMJN were reduced, chemotherapy being continued when paper was published [31]; one case in Croatia, mentioned between other malignancies signaled by SMJN (2021) [32]. The authors considered the Romanian endometrioid endometrial carcinoma case, registered in 2018/2019 to be the 36th case of SMJN in the literature of different languages.
3.2 Pathomechanisms of Secondary Tumor/Metastases in Umbilicus
The mechanisms of tumor dissemination to the umbilicus are poorly understood, being proposed since long time some routes for the spread of the malignant cells in conjunction to the special and unique place of the umbilical skin and the periumbilical areas- the proximity of the abdominal and pelvic organs [22,33], and their natural evolution after birth. There are discussed as possible mechanisms of dissemination:
• direct or contiguous transperitoneal spread via the lymph vessels along obliterated umbilical vein, or
• hematogenic dissemination through access to venous or arterial channels of the anterior abdominal wall, which vary by patient anatomic peculiarities
• via embryologic remnants in the abdominal wall as falciform ligament, or median umbilical ligament, or a remnant of the umbilical channel.
After umbilical metastases one may discover superficial axillary, and inguinal nodes, and deep para-aortic, internal mammary nodes involvement, which is explained in connection to the central point of intersections at umbilical level of the deep and superficial lymph system [24]. The authors suppose that the parietal and umbilical invasion in the Romanian patient are through vascular spread, being discovered vascular neoplastic thrombus in the uterus and in SMJN (Figure 2, A and B), and also positive left external iliac nodes.
3.3 Diagnosis Accuracy: Positive and Differential Diagnosis
SMJN discovery imposes to think first to a metastasis, and secondary to proper area lesions, as benign, which are called “Pseudo Sister Joseph Nodule” by Amaro R, et al. [34] with 57% incidence, and malign, melanoma being the most common primary umbilical malignancy in cases from Duke University [22]. One must think that some of benign umbilical conditions may hide an abdominal malignancy, as it was discussed previously about umbilical hernia associated to endometrial carcinoma [15,30,35].
3.3.1 Dermoscopic examinations: Recently the benign umbilical tumors (19 cases) were compared (clinical and dermoscopic) to “SMJ pure nodule” (30 cases: 28 from PubMed, plus 2 from South Korea) by South Korea dermatologists [21]. There were analyzed in the “benign umbilical tumor”group: dermatofibroma (5/19), keloid (3/19), and soft fibroma (3/19), and SMJN, using a dermoscope (DermLite II Pro HR equipment; 3Gen LLC., Dana Point, CA, USA;×10 magnification) attached to a digital camera (Sony Cyber-shot DSC-W290; Sony Corporation, Tokyo, Japan; ×5 optical zoom, 12.1 megapixels); the digital obtained images were compared to final pathological analyses Table 2.
| Detail of comparison | Sister Mary Joseph Nodule | Benign umbilical tumors |
|---|---|---|
| Colors | Erythematous color, violet, may be white, or skin tone; black is rare; Oozing or ulceration on the surface, and nearby satellite lesions, in the vicinity of the umbilical nodule |
Different colors: red or dusky red, brown to black, and flesh colored. Exhibit characteristic surface changes (e.g., verrucous changes in epidermal nevi and verrucae). |
| Consistency | A firm and fixed consistency (93.3%; 28/30), indicating packed cellularity and invasion into the adjacent tissues, both of which are characteristics of malignancy |
None of the benign tumors had a fixed consistency |
| Pattern | Polymorphous vascular pattern and a white or milky-red, amorphous area, which is considered a pathognomonic sign of malignant neoangiogenesis (primitive or metastatic as SMJN) |
Different dermoscopic patterns: pigment networks with white areas (dermatofibromas), thrombosed capillaries (verrucae), and the "pore sign" (epidermal cysts) |
| Table 2: SMJN's dermoscopic comparison to benign umbilical tumors. Modified from Ha D-L, Yang M-Y, Shin J-O, Kim H-S, Kim M-B, et al. (2021) [21]. | ||
Table 2: SMJN’s dermoscopic comparison to benign umbilical tumors. Modified from Ha D-L, Yang M-Y, Shin J-O, Kim H-S, Kim M-B, et al, (2021) [21].
3.3.2.Imagistic studies: Besides these details one must continue the assessments with complete hematologic picture, and imagistic techniques: ultrasound, CT, MRI of thorax, abdomen, pelvis, head and neck, all being individualized. A special remark regards the soft tissue sonography at umbilicus level, where one may depict hypoechoic masses with irregular margins and small internal hyperechoic foci, and further evaluation may reveal disseminated malignancy, so the umbilical nodule was named “ a tip of an iceberg” [4].
3.3.3 Cytologic studiess: The cytology assessment from fine needle aspiration or core biopsy of a SMJN are simple, fast, accurate and cost efficient [36,37], permitting the pathologist to observe the changes in cell structure and morphology. Sometimes is necessary much attention to avoid bowel lesion if a hernia is inside the”pseudo SMJN”, and under mobile ultrasound machine one may succeed. Sometimes the source may not be determined, and immunehistochemistry can help [31], sustaining the malignancy of primary umbilical lesions in 12% cases, and the remaining are metastases [22]. In the Romanian case it was done excision- biopsy by a high trained dermatologist, without pathological depiction of primary malignancy.
3.3.4 Pathological studies of primary tumor and SMJN: A metastatic umbilical tumor usually reveals an adenocarcinoma, but one may register sarcoma, mesothelioma, and melanoma. The Romanian final diagnosis was a stage IVb malignancy in endometrium that determinate the SMJN, as umbilical metastasis.
When Romanian case was treated and registered, the International Society of Gynecological Pathologists [38] described low grade and high grade endometrial cancers, according to molecular analysis: low grade or type 1 or endometrioid endometrial carcinoma, or the “indolent” cancer, which is estrogen dependent, has “atypical endometrial hyperplasia”, as precursor (23% cases progress to endometrioid adenocarcinoma), a monoclonal lesion, with microsatellite instability, and ras and PTEN mutations [39-41], and PTEN gene loss in up to 65% cases with Endometrial Intraepithelial Neoplasia (EIN) and in 85% cases with endometrioid carcinoma [42] with estrogen and progesterone receptors at immuno- histochemistry analysis [43], discovered more frequent in white, Caucasian, and younger women. This type of endometrioid endometrial carcinoma was complicated by SMJN after 12 years in the Bulgarian case [33]. high grade endometrial carcinomas are represented by previous FIGO grade 3 endometrioid carcinoma, serous endometrial carcinoma, clear cells endometrial carcinoma, undifferentiated carcinoma, and carcinosarcoma. They are non-estrogen dependent, more aggressive, with atrophia or with a polyp rather than hyperplasia as precursor, and they are nonresponsive to progestins [44]. They contain p53 mutations and abnormal accumulation of p53 protein, and absence of ERs, PRs [43,45]. The literature presents the majority of cases of SMJN to be associated to high grade endometrial carcinomas. The Romanian case may be considered a low grade endometrial carcinoma, being an endometrioid moderate differentiated carcinoma, with squamous component, with presence of ERs and PGR in high percentages- as immunohistochemistry showed in uterus and umbilical metastases, and according these predictive molecular factors one registered good response to progestin, Megesin® in the first 2 years after radical surgical procedure. SMJN relapsed after initial dermatologist’s ablation, but not after radical abdominal extrafascial total utero-adnexal ablation, plus radiotherapy.
Prognosis in Sister Mary Joseph Nodule
One discusses a poor prognosis in cases with SMJN, which are stage IVb malignancies, a short survival duration, with a median period of 28 weeks (range, 2 to 64 weeks), even post surgery deaths [20,28], and SMJN recurrence after complete excision in Tanzania cases [20], but also in USA Afro-American case with impossible complete excision of uterus and invaded cervix [28], depending of primary organ tumor’s origin, type/degree of differentiation, possibilities of radical surgery with complete ablation of primary organ; patient’s comorbidities- number and severity, response to chemo- and hormone therapy, as in the Romanian case, medical capacities of oncologic monitoring, and case’s compliance. Usually endometrioid endometrial carcinoma has a good prognosis, with long term survival in comparison to ovarian and digestive cancers
Conclusion
mass, being more frequent in women, and in elder patients one must think first at a metastasis from an abdominal organ, the endometrium being after the ovary, but there are population/ racial variations. The sonography, CT, MRI and molecular markers are useful tools for diagnosis, the golden standard being the pathological diagnosis of the primary tumor, with immunehistochemistry, for adequate chemotherapy/ hormonotherapy. The Romanian reported SMJN was associated to a medium and weak differentiated endometrioid endometrial carcinoma with squamous component and desmoplazic reaction, with cervical stroma and external myometrial half invasion, a low grade type of endometrial malignancy, with long duration from onset to SMJN appearance, final diagnosis was not suggested by SMJN biopsy. According to steroid hormones receptors presence in primary endometrial carcinoma one may discuss a patient’s favorable prognosis, different to other cases from literature.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Acknowledgement
The authors express their thanks to the patient and her family for permission to publish this case report.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
References
1. Constantine, D.G., Kessler, G., Graham, S., Golstein, R.S. (2019). Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J Womens’ Health (Larchmt), 28(2), 237-243.
2. Viau, M., Renaud, M.C., Grégoire, J., Sebastianelli, A., Plante, M. (2017). Paraneoplastic syndromes associated with gynecological cancers: A systematic review. Gynecologic Oncology, 146 (3), 661-671.
3. Schadt, R.C. (2016). The cutaneous manifestations of gastrointestinal malignancy. Semin.Oncol, 43(3), 341-346.
4. Wronski, M., Klucinski, A., & Krasnodebski, W. (2014). Sister Mary Joseph nodule: a tip of an iceberg. J Ultrasound Med, 33(3), 531-534.
5. Kirshtein, B., Meirovitz, M., Okon, E., Piura, B. (2006). Sister Mary Joseph’s nodule as the first presenting sign of primary fallopian tube adenocarcinoma. J Minim Invasive Gynecol, 13(3), 234-236. 6. Petch, S., Sobota, A., & Abu Saadeh, F. (2019). Sister Mary Joseph nodule: an unusual site for endometrioid cancer metastasis. BMJ Case Rep, 12(5), e229187
7. Renner, R., & Sticherling, M. (2007). Sister Mary Joseph’s nodule as a metastasis of gallbladder carcinoma. Int J Dermatol, 46(5), 505-507.
8. Levine, D., Miller, S., Al-Dawsari, N., Barak, O., Gottlieb, A.B. (2010). Paraneoplastic dermatoses associated with gynecologic and breast malignancies. Obstet Gynecol Surv, 65(7), 455-461.
9. Dar, I.H., Kamili, M.A., Dar, S.H., & Kuchhai, F.A. (2009).Sister Mary Joseph nodule: an unusual case report with review of literature. J Res Med Sci, 14(6), 385-387.
10. Ito, S., Yoshitomi, H., Pak, M., Kawahara, H., Tanabe, K., et al. (2013).Trousseau syndrome with nonbacterial thrombotic endocarditis in a patient with uterine cancer. Intern Med, 52(12), 1353-1358.
11. Hegazy, A.A. (2016). Anatomy and embryology of umbilicus in newborns: a review and clinical correlations. Front Med, 10, 271-277.
12. FIGO Committee on Gynecologic Oncology (2014). FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 125(2), 97–98.
13. Bailey, H. (1949). Demonstrations of Physical Signs. In 11th ed. Clinical Surgery, Baltimore, Williams and Wilkins, cited by Powell JL.
14. Powell, J.L. (2011). Powell’s pearls: eponyms in medical and surgical history. Sister Joseph’s nodule; Sister Mary Joseph (1856-1939) J Surg Educ, 68(5), 442-443.
15. Piura, B., Meirovitz, M., Bayme, M., Shaco-Levy, R. (2006). Sister Mary Joseph’s nodule originating from endometrial carcinoma incidentally detected during surgery for an umbilical hernia: a case report. Arch Gynecol Obstet, 274(6), 385-388.
16. Fukuda, H., & Saito, R. (2006). A case of Sister Mary Johnson nodule from prostatic cancer. The Journal of Dermatology, 33(1), 46-51.
17. Sina, B., Deng, A.J. (2007). Umbilical metastasis from prostate carcinoma (Sister Mary Joseph’s nodule): a case report and review of literature .Cutan Pathol, 34(7), 581- 583.
18. Davar, S., Hanna, D. (2012). Sister Mary Joseph’s nodule. Cutan Med Surg, 16(3), 201-204.
19. Khan, K., Bagchi, D. (2011). Squamous cell carcinoma arising in a testicular teratoma and presenting as Sister Mary Joseph nodule. J Surg Tech Case Rep, 3, 99-101.
20. Chalya, P.L., Mabula, J.B., Rambau, P.F., Mc Hembe, M.D. (2013). Sister Mary Joseph’s nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol, 2013, 11:151.
21. Ha D-L, Yang M-Y, Shin J-O, Kim H-S, Kim M, et al. (2021) Benign Umbilical Tumors Resembling Sister Mary Joseph Nodule.Clin Med Insights Oncol, 24;15: eCollection 2021
22. Papalas, J.A., Selim, M.A. (2011). Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol, 15(4), 237-242.
23. Heller, D.S. (2012). Lesions of the umbilicus: what the minimally invasive gynecologic surgeon needs to know about the belly button. J Minim Invasive Gynecol, 19, 680- 683.
24. Dubreuil, A., Dompmartin, A., Barjot, P., Louvet, S., Leroy, D. (1998). Umbilical metastasis or Sister Mary Joseph’s nodule. Int J Dermatol, 37, 7-13.
25. Piura, B. (2006). Umbilical metastasis.Sister Mary Joseph’s nodule. Harefuah, 145(7), 505-509.
26. Poncelet, C., Bouret, J.M., Boulay, I., Tsatsaris, V., Ferrand, J., et al. (1996). Métastase ombilicale d’un adénocarcinome de l’endomètre: “Sister (Mary) Joseph’s nodule”. Revue de la littèrature [Umbilical metastasis of an endometrial adenocarcinoma: “Sister (Mary) Joseph’s nodule”. Review of the literature]. Journal de gynecologie, obstetrique et biologie de la reproduction, 25(8), 799-803. 27. Fairchild, A., Janoski, M., Dundas, G. (2007). Sister Mary Joseph’s nodule. CMAJ, 176(7), 929-930.
28. Sengupta, S., Church, E., Chia, K.V. (2009). Sister Mary Joseph’s nodule: recurrent endometrial adenocarcinoma presenting as an umbilical metastasis. J Obstet Gynaecol 29, 170-171.
29. Nolan, C., Semer, D. (2012). Endometrial cancer diagnosed by Sister Mary Joseph nodule biopsy: Case report. GynecolOncol Case Rep, 2, 110-111.
30. Rahman, M.T., Nakayama, K., Rahman, M., Nakayama, N., Ishikawa, M., et al. (2012). Sister Mary Joseph’s nodule associated with rare endometrial squamous cell carcinoma. Archives of gynecology and obstetrics, 286(3), 711-715.
31. Li, Y., Guo, P., Wang, B., Jia Y.T. (2019). Sister Mary Joseph’s nodule in endometrial carcinoma: A case report. World J Clin Cases, 7(20), 3358-3363.
32. LugoviÄ?-MihiÄ?, L., Krišto, M., Špoljar, S., Novak-BiliÄ?, G., Šitum, M., et al. (2021). Can skin be a marker for internal malignancy? Evidence from clinical cases .Acta Clin Croat, 60 (4), 711-721.
33. Lookingbill, D.P., Spangler, N., Helm, K.F. (1993). Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol, 29, 228-236.
34. Amaro, R., Goldstein, J.A, Cely, C.M. (1999). Pseudo Sister Mary Joseph’s nodule. Am J Gastroenterology, 11, 19491950.
35. Poncelet, C., Bouret, J.M., Boulay, I., Tsatsaris, V., Ferrand, J., et al. (1996). Métastase ombilicale d’un adénocarcinome de l’endomètre: “Sister (Mary) Joseph’s nodule”. Revue de la littèrature (Umbilical metastasis of an endometrial adenocarcinoma: “Sister (Mary) Joseph’s nodule”. Review of the literature). Journal de gynecologie, obstetrique et biologie de la reproduction, 25(8), 799-803. 36. Galvan, G. (1999). Sister Mary Joseph’s nodule. Its clinical significance and management”. Anales de Medicina Interna, 16, 365-370.
37. Iavazzo, C., Madhuri, K., Essapen, S., Akrivos, N., Tailor, A., et al. (2012). Sister Mary Joseph’s Nodule as a First Manifestation of Primary Peritoneal Cancer. Case reports in obstetrics and gynecology, 2012, 467240.
38. Rabban, J.T., Gilks, C.B., Malpica, A., Matias- Guiu, X., Mutter, G.L., et al. (2019). Issues in the Differential Diagnosis of Uterine Low-grade Endometrioid Carcinoma, Including Mixed Endometrial Carcinomas: Recommendations from the International Society of Gynecological Pathologists. Int J Gynecol Pathol, 38 (Suppl), S25-S39.
39. Levine, R.L., Cargile, C.B., Kurman, R.J., Ellenson, LH., et al. (1998). PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma.Cancer Res, 58, 3524-3528.
40. Mutter, G.L. (2000). Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol, 76, 287-290.
41. Mutter, G.L. (2001). PTEN, a protean tumor suppressor. Am J Pathol, 158, 1895-1898.
42. Mutter, G. L., Zaino, R. J., Baak, J. P., Bentley, R. C., Robboy, S. J. (2007). Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists, 26(2), 103-114.
43. Sherman M. E. (2000). Theories of endometrial carcinogenesis: a multidisciplinary approach. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc, 13(3), 295-308.
44. Kurman, R.J., Kaminski, P.F., Norris, H.J. (1985). The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer, 56(2), 403- 412.
45. Zheng, W., Khurana, R., Farahmand, S., Wang, Y., Zhang, Z. F., et al. (1998). p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. The American journal of surgical pathology, 22(12), 1463-1473.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



