Case Report - (2023) Volume 8, Issue 8
Significance of Urine pH in Predicting Renal Outcomes for Patients with Rhabdomyolysis
2Department of Nephrology and Hypertension, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Glickman Urological & Kidney Institute Cleveland Clinic, Cleveland Ohi, USA
Received Date: Aug 08, 2023 / Accepted Date: Aug 15, 2023 / Published Date: Aug 28, 2023
Abstract
Acute kidney injury (AKI) is a common complication of rhabdomyolysis. Urine pH is important in predicting the occurrence of AKI. Acidic environment plays a major role in incidence of AKI. In this case report of 32 patients with rhabdomyolysis, majority of patients with urine pH<6.5 developed AKI whereas no renal dysfunction was found in patients with urine pH>7.0, regardless of levels of creatinine kinase. Low urine pH and intratubular acidosis facilitates higher concentration of myoglobin in the tubules as well as precipitation of myoglobin and uric acid casts that results in tubular obstruction and renal dysfunction. Myoglobin has direct nephrotoxic effect in aciduria. Acidification of body fluids and aciduria are independent risk factors for acute kidney injury (AKI) in patients in the presence of met-hemoglobinuria, well documented in patients before and after coronary artery bypass graft surgery. There is, however, lack of literature regarding the role of rhabdomyolysis in predicting renal outcomes in the presence of acidosis and aciduria. Animal studies have shown that under acidic environment, myoglobin is more nephrotoxic than under alkaline conditions, however this has not been documented in humans. This is, to the best of our knowledge, the first study to correlate this phenomenon in humans. This study has wider application in patients with rhabdomyolysis. Early recognition of urinary pH may predict outcome in patients with rhabdomyolysis induced AKI.
Keywords
Rhabdomyolysis, Urine pH, Rhabdomyolysis, Aciduria, Bicarbonate
Statement of Ethics
This study protocol was reviewed and the need for approval was waived by Western IRB. Thus, an exemption was issued by the above organization. Ethical approval is not required for this study in accordance with local and national guidelines.
Written informed consent was obtained from all patients for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
None of the authors declare any conflict of interest.
Funding Sources
Funding Sources No funding was obtained for this study, preparation of data or the manuscript.
Author Contributions
Sushil K. Mehandru: Conceptualization and designing.Preparation of initial draft.
Supreet Kaur: Preparation of the body of the manuscript. Literature research.
Avais Masud: Data collection Qalb Khan: Data collection and analysis.
Kyrillos Rezkalla: Literature research
Prit Paul Singh: Data collection and analysis. Preparation of tables and figures.
Suhas Patel: Literature research. Data analysis
Tushar Vachharajani: Contribution to analysis. Editing
Arif Asif: Contribution to analysis. Final editing
Data Availability
All data generated or analyzed during the study are included in this article. Further inquiries can be directed to the corresponding author.
References
1. Zager, R.A. (1996) Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 49, 314 -326.
2. Lameire, N., Matthys, E., Vanholder, R., De Keyser, K., Pauwels, W., Nachtergaele, L., & Ringoir, S. (1987). Causes and prognosis of acute renal failure in elderly patients. Nephrology Dialysis Transplantation, 2(5), 316-322.
3. McMahon, G.M., Zeng, X., & Waikar, S.S. (2013). A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA internal medicine, 173(19), 1821- 1827.
4. Efstratiadis, G., Voulgaridou, A., Nikiforou, D., Kyventidis, A., Kourkouni, E., & Vergoulas, G. (2007). Rhabdomyolysis updated. Hippokratia, 11(3), 129.
5. Visweswaran, P., & Guntupalli, J. (1999). Rhabdomyolysis. Critical care clinics, 15(2), 415-428.
6. Woodrow, G., Brownjohn, A.M., & Turney, J.H. (1995). The clinical and biochemical features of acute renal failure due to rhabdomyolysis. Renal failure, 17(4), 467-474.
7. Tumer, N.B., & Gunaydin, S. (2019, November). Preoperative Urinary pH is Associated with Acute Kidney Injury After Cardiac Surgery in Non-Diabetic Patients. In The Heart Surgery Forum (Vol. 22, No. 6, pp. E456-E461).
8. Haase, M., Haase-Fielitz, A., Bagshaw, S.M., Ronco, C., & Bellomo, R. (2007). Cardiopulmonary bypass-associated acute kidney injury: a pigment nephropathy?. Acute Kidney Injury, 156, 340-353.
9. Clyne, D.H., Kant, K.S., Pesce, A.J., & Pollak, V.E. (1979). Nephrotoxicity of low molecular weight serum proteins: physicochemical interactions between myoglobin, hemoglobin, bence-jones proteins and tamm-horsfall mucoprotein. Current Problems in Clinical Biochemistry, (9), 299-308.
10. Milani, D.A.Q. (2021) Urinalysis. Stat Pearls (Internet). Published May 9, 2021.Available from: https://www.ncbi. nlm.nih.gov/books/NBK557685/
11. Vanholder, R., SU, M., Erek, E., & Lameire, N. (2000). Rhabdomyolysis. Journal of the American Society of Nephrology, 11(8), 1553-1561.
12. Miller, M. L. (2013). Clinical manifestations and diagnosis of rhabdomyolysis. Uptodate BN tiêu cÆ¡ vân cấpcó nguy cÆ¡ suy tháºn Äảm bảo hô hấp Äặt catheter Ä?o áp lá»±c tÄ©nh mạch trung tâm (ALTMTT) Truyá»n NaCl 0, 9.
13. Giannoglou, G.D., Chatzizisis, Y.S., & Misirli, G. (2007). The syndrome of rhabdomyolysis: pathophysiology and diagnosis. European journal of internal medicine, 18(2), 90- 100.
14. Warren, J.D., Blumbergs, P.C., & Thompson, P.D. (2002). Rhabdomyolysis: a review. Muscle & nerve, 25(3), 332- 347.
15. Nemiroff, L., Cormier, S., LeBlanc, C., & Murphy, N. (2012). Don’t you forget about me: considering acute rhabdomyolysis in ED patients with cocaine ingestion. Canadian Family Physician, 58(7), 750-754.
16. Kenny, J.E. (2010) Creatine kinase: How much is too much? Available from: https://www.clinicalcorrelations. org/2010/11/03/creatine-kinase-how-much-is-too-much/.
17. Bagley, W., Yang, H., & Shah, K. (2007) Rhabdomyolysis. Int Emergency Med 2, 210-218.
18. Bosch, X., Poch, E., & Grau, J. M. (2009). Rhabdomyolysis and acute kidney injury. New England Journal of Medicine, 361(1), 62-72.
19. Salahudeen, A.K., Wang, C., Bigler, S.A., Dai, Z., & Tachikawa, H. (1996). Synergistic renal protection by combining alkaline-diuresis with lipid peroxidation inhibitors in rhabdomyolysis: possible interaction between oxidant and non-oxidant mechanisms. Nephrology Dialysis Transplantation, 11(4), 635-642.
20. Mathes, D.D., Assimos, D.G., & Donofrio, P.D. (1996). Rhabdomyolysis and myonecrosis in a patient in the lateral decubitus position. The Journal of the American Society of Anesthesiologists, 84(3), 727-729.
21. Holt, S., & Moore, K. (2000). Pathogenesis of renal failure in rhabdomyolysis: the role of myoglobin. Experimental nephrology, 8(2), 72-76.
22. Homsi, E., Barreiro, M. F. F. L., Orlando, J. M. C., & Higa, E. M. (1997). Prophylaxis of acute renal failure in patients with rhabdomyolysis. Renal failure, 19(2), 283-288.
23. Cervellin, G., Comelli, I., & Lippi, G. (2010). Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clinical chemistry and laboratory medicine, 48(6), 749–756.
24. Chatzizisis, Y.S., Misirli, G., Hatzitolios, A.I., & Giannoglou, G.D. (2008). The syndrome of rhabdomyolysis: complications and treatment. European journal of internal medicine, 19(8), 568–574.
25. Bragadottir, G., Redfors, B., & Ricksten, S.E. (2012). Mannitol increases renal blood flow and maintains filtration fraction and oxygenation in postoperative acute kidney injury: a prospective interventional study. Critical care (London, England), 16(4), R159.
26. Melli, G., Chaudhry, V., & Cornblath, D.R. (2005). Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine, 84(6), 377–385.
27. Delaney, K.A., Givens, M.L., & Vohra, R.B. (2012). Use of RIFLE criteria to predict the severity and prognosis of acute kidney injury in emergency department patients with rhabdomyolysis. The Journal of emergency medicine, 42(5), 521–528.
28. de Meijer, A.R., Fikkers, B.G., de Keijzer, M.H., van Engelen, B.G., & Drenth, J.P. (2003). Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive care medicine, 29(7), 1121– 1125.
29. Ron, D., Taitelman, U., Michaelson, M., Bar-Joseph, G., Bursztein, S., & Better, O.S. (1984). Prevention of acute renal failure in traumatic rhabdomyolysis. Archives of internal medicine, 144(2), 277–280.
30. Gabow, P.A., Kaehny, W.D., & Kelleher, S.P. (1982). The spectrum of rhabdomyolysis. Medicine, 61(3), 141–152.
31. Brown, C.V., Rhee, P., Chan, L., Evans, K., Demetriades, D., & Velmahos, G.C. (2004). Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference?. The Journal of trauma, 56(6), 1191– 1196.
Introduction
can develop from variety of causes. Acute renal failure (ARF) develops in patients with complications in days following initial presentation of rhabdomyolysis. Renal injury can vary from mild to severe, with need for renal replacement therapy in some cases. In most of the cases, renal function is restored with hydration. Factors known to contribute to rhabdomyolysisinduced acute renal failure include hypovolemia, acidosis or aciduria, tubular obstruction, and direct tubular toxic effect of myoglobin. Although, elevated plasma creatinine kinase (CK) is the most sensitive finding pertaining to rhabdomyolysis, significance of urine pH cannot be ignored for appropriate and timely management of renal injury. ARF occurs only if a critical mass of muscle becomes necrotic. The degree of rhabdomyolysis that can manifest ranging from a ‘subclinical rise of creatinine kinase to a medical emergency comprising interstitial and muscle cell edema, contraction of intravascular volume, and pigment induced ARF. Adverse effects of rhabdomyolysis on kidneys can be short or long term, the concern remains how to identify possible renal involvement as well as implement renal protective measures. Although, co-morbid conditions play a crucial role, early intervention can avoid any renal injury or long term effects of it.
Our present study reveals the significance of urine pH in predicting renal outcomes in patients with rhabdomyolysis. Urine pH 7.0. Early diagnosis and treatment of AKI in rhabdomyolysis patients improved overall prognosis. Rhabdomyolysis is one of the leading causes of ARF [1]. The prognosis of rhabdomyolysisassociated ARF is relatively benign [2].
A retrospective study conducted by Tumer et al shows conclusions similar to ours, however their study focused on nephrotoxicity of met-hemoglobin and significance of urine pH, before and after CABG [3], whereas our present study is about myoglobin contributing to renal failure. The nephrotoxicity is caused by free iron and generation of reactive oxygen species leading to occlusion of tubules by met hemoglobin casts. Low preoperative urine pH (<5.5) resulted in severe AKI and increased rate of morbidity and mortality after isolated coronary artery bypass graft (CABG). No patient developed AKI in the group with preoperative urine pH of over 7.0. Cardiopulmonary bypass results in the destruction of cells, primarily red blood cells (RBCs) and hemolysis occurs, due to this damage, there is free hemoglobin and iron in plasma. Hemolysis results in the release of free hemoglobin that forms casts with methemoglobinuria and causes tubular obstruction and ultimately renal cellular necrosis [3]. Free iron is nephrotoxic; it results in generation of reactive oxygen species (ROS) thereby resulting in acute tubular necrosis (ATN), due to occlusion of renal tubules by met hemoglobin casts [4]. In rhabdomyolysis the amount of myoglobin delivered to the proximal tubule cells overwhelms their ability to convert iron to ferritin, resulting in intracellular ferrihemate accumulation [5]. Acidity alters myoglobin from an anion to a cation, increasing its glomerular filtration and conversion to acid hematin, which reacts with tubular protein and precipitates in proximal tubules [6]. Three types of low molecular weight serum proteins, myoglobin, hemoglobin and BENCE-JONES proteins are associated clinically with acute renal failure. All have isoelectric points which render them anionic at blood pH but cationic in the distal nephron under conditions of aciduria [6]. These data suggest that protein pl and urine pH are important in determining nephrotoxicity; a mechanism by which these low molecular weight serum proteins and TAMM-HORSFALL proteins interact in the distal nephron to initiate acute renal failure is postulated [6].
Case Report
| Patient | CPK | Urine Sodium | GFR | Urine Myoglobin | Urine pH | Serum Na |
|---|---|---|---|---|---|---|
| 1 | 45,480 | 65 | 10 | (+) 52.3 | 6.5 | 135 |
| 2 | 8,690 | 26 | 9 | (+) | 5.0 | 133 |
| 3 | 6,164 | 111 | 60 | (+) 9.944 | 6.0 | 141 |
| 4 | 3,456 | 46 | 30 | (-) | 6.0 | 141 |
| 5 | 47,320 | 110 | 37 | (-) | 6.0 | 141 |
| 6 | 17,808 | 18 | 28 | (-) | 5.0 | 147 |
| 7 | 57,000 | 556 | 60 | (-) | 6.0 | 141 |
| 8 | 7,330 | 46 | >60 | (+) 28,000 | 5.0 | 136 |
| 9 | 450 | 46 | 56 | (-) | 7.0 | 136 |
| 10 | - | <10 | 48 | (-) | 5.0 | 136 |
| 32 | 546 | - | 31 | (-) | 5.0 | 136 |
Statistical Analysis
All differences among the groups were examined for statistical significance using a two-tailed Student t test and one-way ANOVA. GraphPad Prism 374 software was used to perform statistical analysis. P<0.05 was considered significant. **P 0.0029.
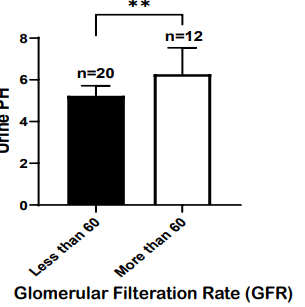
Figure 1: High urine PH as a predictor of acute kidney injury in rhabdomyolysis patients.
32 patients with rhabdomyolysis were screened for their Urine PH and glomerular filtration rate (GFR). Urine PH was shown for patients with GFR of less than 60, indicative of AKI, or more than 60. Patients with GFR of less than 60 had lower PH (n=20) or acidic urine as compared to patients with normal GFR of more than 60. Summary data ± SD are shown; Unpaired T-test, 2 tail. **P 0.0029.
Results
The study was conducted to determine correlation between Urine PH and glomerular filtration rate (GFR), where later is predictive of Acute kidney injury (AKI). The study included 32 patients with the rhabdomyolysis and were screened for their Urine PH and glomerular filtration rate (GFR) to assess acute kidney injury. The study found that patients with glomerular filtration rate of less than 60 (n=20), which predicts acute kidney failure, had lower urine pH (5.237 ± 0.482 (mean ± SD)) or acidic urine as compared to heathy individuals (n=12) with GFR of more than 60 (6.269 ± 1.268 (mean ± SD)) (shown in Figure 1). The significant correlation (P=0.0029) between low urine pH and higher incidence of AKI or renal dysfunction was observed in the patients with rhabdomyolysis. The study did not find a direct relationship between urine pH or GFR and urine myoglobin levels (data not shown).
Discussion
Rhabdomyolysis is one of the leading causes of ARF [1,7]. High rate of rhabdomyolysis incidence is from compartment syndrome 41.2%, sepsis 39.3%, following cardiac arrest 58.5%, whereas the lowest rate of rhabdomyolysis is from events such as myositis 1.7%, exercise 3.2%, and seizures 6.0% [8]. Independent predictors are age, female sex, cause of rhabdomyolysis, value of initial creatinine; CPK, phosphate, calcium, and bicarbonate levels [8].
Most cases of rhabdomyolysis in hospital patients are nontraumatic, they are more frequently consequence of seizures, alcohol abuse, exercise-induced or muscle compression as a result of coma. Drugs may also result in rhabdomyolysis, with cholesterol medications and psychotropic drugs being the main offenders.
In rhabdomyolysis one of the components released is myoglobin, an 18,800 Dalton oxygen carrier. It resembles hemoglobin but contains only one heme moiety. Normally myoglobin is loosely bound to plasma globulins and only small amount reaches the urine. When massive amount of myoglobin is released, the binding capacity of plasma protein is exceeded, myoglobin is then filtered by the glomeruli and reaches the tubules, where it may cause obstruction leading to renal dysfunction [1].
Lower urine pH and intra tubular acidosis facilitates intratubular precipitation of myoglobin and uric acid, leading to cast formation. Pathophysiology of myoglobinuric ARF has been studied in animal models of glycerol induced ARF. The main pathophysiologic mechanisms in these studies are vasoconstriction, intraluminal cast formation, and direct hemeprotein-induced nephrotoxicity. High rate of generation and urinary excretion of uric acid further contributes to tubular obstruction by uric acid casts (shown in Figure 2).
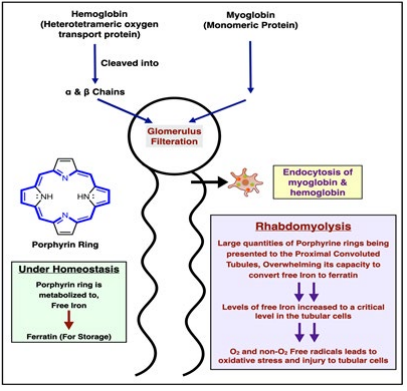
Figure 2: Rhabdomyolysis.
Myoglobinuria does not occur without rhabdomyolysis. Myoglobin is rapidly and unpredictably eliminated by hepatic metabolism. Therefore, tests for myoglobin in plasma or urine are not sensitive diagnostic procedures. Myoglobin is usually filtered through glomeruli and reabsorbed in the proximal tubule by endocytosis, however when rhabdomyolysis occurs there is an excess of myoglobin, which overloads the ability of proximal tubule cell to convert iron to ferritin, which then results in intracellular ferrihemate accumulation (figure 1) [5]. Since iron can donate and accept electrons as well as having the ability to generate free radicals, low urine pH can lead to metabolic acidosis. This process puts oxidative stress injury to the renal cells. This acidosis results from release of intracellular phosphate and sulfate [9]. Alkaline conditions prevent this effect by stabilizing the reactive ferryl-myoglobin complex.
Our study was conducted with the aim to analyze the effect of acidic urine on renal function in patients with rhabdomyolysis.
Urine pH is a vital piece of information and provides insight into tubular function [10]. Major factor favoring precipitation of myoglobin and uric acid is a low pH of tubular urine, which is common because of underlying acidosis [11]. Free iron has been found to be one of the factors causing nephrotoxicity secondary to met-hemoglobinuria [3] and myoglobinuria. All these factors discussed in previous studies and evidence from our own research has led us to believe that urine pH is directly involved in renal damage secondary to myoglobinuria.
Lower urine pH is mostly seen in patients with diabetes mellitus, metabolic syndrome, obesity, and chronic kidney disease (CKD). It is suggested that low urinary pH is a consequence of acidification of body fluids. Low urinary pH often found in metabolic syndrome is an independent risk factor for AKI occurrence after cardiac surgery. Patient with obesity and BMI >30 Kg/m2 have lower urinary pH that is statistically significant and had high incidence of AKI post CABG [3].
The unreliable nature of urine myoglobin cannot be ignored. Pigmenturia will be missed in rhabdomyolysis if filtered load of myoglobin is insufficient or has largely resolved before the patient seeks medical attention due to rapid clearance [10]. Routine urine testing for myoglobin by urine dipstick evaluation may be negative in up to half of the patients with rhabdomyolysis [11]. This was the case in our study as well, more than half of our patients tested negative for urine myoglobin. Muscle breakdown is accompanied by visible myoglobinuria when urinary myoglobin excretion exceeds 250 ug/mL normal (100g of muscle [12]. Myoglobin is not measured directly in urine or plasma;measurement of serum myoglobin actually has a low sensitivity for the diagnosis of rhabdomyolysis because serum myoglobin levels peak earlier than serum CK levels, and it has a short halflife and unpredictable mechanism [13].
It is not uncommon for CPK levels to remain elevated in the absence of myoglobinuria [13]. In rhabdomyolysis myoglobin appears in the plasma before CPK elevation occurs and disappears while CPK is still elevated and rising. Therefore, there is no CPK threshold for when myoglobin appears. As mentioned above, rhabdomyolysis does not occur unless CPK is elevated five times or more above the upper limit of normal [10]. Creatinine kinase is more reliable than myoglobin in assessing the presence and intensity of damage to the muscle. Hypovolemia and aciduria are felt to be key pathophysiological events leading to AKI in the setting of muscle breakdown [14]. Damage to the kidney is mediated by heme protein released from myoglobin [15].
There are four converging pathways by which heme protein harms the kidneys:
1. Renal vasoconstriction
2. Cytokine activation
3. Precipitation of Tamm-Horsefall protein at an acid pH with subsequent cast nephropathy
4. Acid sensitive renal free radical production [15]
Similar conclusions were reached by another study by Nemiroff et al, suggest that although exact mechanism of rhabdomyolysis induced renal dysfunction is unclear, it appears that intrarenal vasoconstriction, direct and indirect ischemic tubule injury, and obstruction in the distal tubules from concentrated myoglobin are all important contributing factors [13] (shown in Figure 3). Cytotoxicity might be due to uncontrolled leakage of reactive oxygen species after cellular release of myoglobin and free radicals that cause tissue injury [16].
Uric acid is released from nucleotides and forms crystal deposits in the presence of acidic environment causing tubular cell destruction and AKI. Uric acid casts are also formed in the tubules in acidic environments resulting in tubular obstruction.
Due to many liters of fluids sequestrated in injured muscle, patients with rhabdomyolysis develop profound volume depletion. Consequently, homeostatic mechanisms, such as renin-angiotensin, aldosterone and vasopressin systems, are activated leading to renal vasoconstriction.
Various cytokinin induced in rhabdomyolysis have also been shown to have similar effects on renal profusion [15]. Because myoglobin becomes concentrated in aciduria, it precipitates with Tamm-Horsefall protein and also induces free radical production [1].
Given the aforementioned mechanisms of AKI, evidence of CPK elevation should lead in attempts to protect the kidneys. Treatment should include reversal of fluid deficit with or without urinary alkalization. Despite protective effects of urinary alkalization on experimental models of heme protein nephrotoxicity [17] and similar positive reports from various case series [18], evidence from randomized control trials is lacking [14].
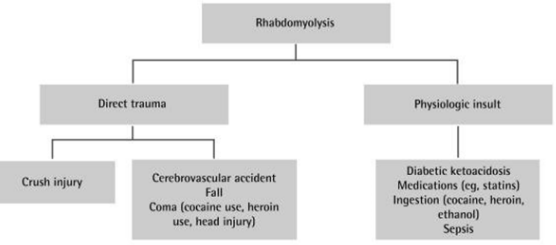
Figure 3: Differential diagnosis for rhabdomyolysis. Courtesy: Nemiroff et al.
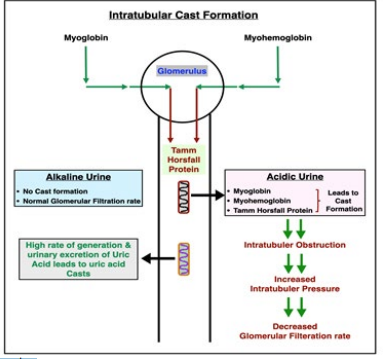
Figure 4: Intratubular cast formation
Myoglobin is easily filtered through the glomerular basement membrane. Water is progressively reabsorbed in the tubules, and the concentration of myoglobin rises proportionally, until it causes obstructive cast formation [19]. This has been graphically explained by the authors of this study in figure 2. The high rate of generation and urinary excretion of uric acid further contributes to tubular obstruction with uric acid casts. Another factor favoring precipitation of myoglobin and uric acid is a low pH of tubular fluid, which is common because of underlying acidosis. The degradation of intra tubular myoglobin results in release of free iron, which catalyzes free radical production and further enhances ischemic changes [1]. Even without invoking release of free iron the heme center of myoglobin will initiate lipid Peroxidation and renal injury [20].
Alkaline conditions prevent the effect of free iron by stabilizing the reactive ferryl myoglobin complex. During rhabdomyolysis, extreme quantities of creatinine kinase from skeletal muscle (CK-MM) are released and peak concentrations of 100,000 IU/ ML or more are not unusual [19]. Because overall degradation and removal are slow, the concentration of CK remains elevated much longer and in a more consistent manner than that of myoglobin [19]. Consequently, a retrospective study of 24 patients demonstrated that augmentation with mannitol and bicarbonate have no benefit over aggressive fluid resuscitation with saline alone [21].
Renal injury with high serum CPK values becomes a concern when levels of CPK reach 5,000 IU/L in the presence of comorbid conditions such as volume depletion, sepsis, or acidosis, otherwise values of 20,000 IU/L may be tolerated without untoward event [14]. The key pathophysiological events are volume depletion and aciduria, which should be corrected immediately and primarily with ample intravenous normal saline and secondarily with urine alkalinization [14] (shown in Figure 4).
There is limited time to prevent renal injury, perhaps as little as 6 hours after occurrence of rhabdomyolysis. It is suggested that if myoglobinuria is present, alkalinization of urine through intravenous sodium bicarbonate solution should be initiated, with the objective of reaching a urine pH greater than 6.50 and a serum pH between 7.40 and 7.45 [22,23 ]. Whereas urinary alkalinization of unproven benefit has also been found in literature. The risk of continued sodium bicarbonate administration far outweighs little change of benefit at that point. The addition of mannitol to the fluid regimen serves several purposes: (1) mannitol increases renal blood flow and GFR; (2) mannitol is an osmotic agent that attracts fluid from the interstitial compartment, thus counterbalancing hypovolemia and reducing muscular swelling and nerve compression; (3) mannitol is an osmotic diuretic that increases urinary flow and prevents obstructive myoglobin casts; and (4) mannitol scavenges free radicals (19).
Another study echoed similar results, to evaluate in more detail the potential beneficial effects of mannitol for treatment of AKI in the perioperative setting, the aim was to evaluate the effects of mannitol on GFR, renal blood flow (RBF), renal oxygen consumption (RVO2), and the renal oxygen supply/demand relation in patients with early, ischemic AKI after complicated cardiac surgery Mannitol in the treatment of postoperative ischemic AKI
• Causes renal vasodilation with a 12% increase in renal blood flow
• Redistributes systemic blood flow to the kidneys
• Maintains renal filtration fraction (that is, causes a balanced increase in GFR and renal plasma flow)
• Maintains the renal oxygen supply/demand relation (that is, the increase in renal blood flow was matched by a proportional increase in renal oxygen consumption) [24].
Acute kidney injury is a feared and common complication of rhabdomyolysis, occurring in 13 to 50% of patients [25,26]. reported mortality rate is as high as 59% in critically ill patients [27]. The degree of CPK elevation is often used clinically as a marker of disease severity but has been reported to have a weak correlation with risk.
In one study, investigators compared the clinical outcomes of two groups of patients who developed crush syndrome during building collapses. All seven patients in this group that had intravenous fluid delayed for more than 6 hours required dialysis, whereas none of the seven patients with similar injuries in the group that received intravenous fluids at time of extrication developed AKI [28].
A retrospective study of 24 patients demonstrated that augmentation with mannitol and bicarbonate may have no benefit over aggressive fluid resuscitation with saline alone [21]. Although, there are no randomized controlled studies that prove the yield of mannitol in this scenario and some other studies have found no benefit at all [29]. However large volume saline repletion without alkalization raises the risk of hyperchloremic acidosis and may perpetuate kidney injury [14]. In a recent NEJM review, Bosch et al. recommend both normal saline and sodium bicarbonate in patients with metabolic acidosis [16].
It has now been estimated that approximately one third of the patients with rhabdomyolysis develop acute renal failure [30] and that this condition accounts for approximately 10% to 15% of acute renal failure in hospitalized patients in the United States [1]. Studies have suggested that there is limited time to prevent renal injury perhaps as little as six hours after occurrence of rhabdomyolysis. However, if kidney injury is already established continuing to force intravenous fluids into the patient with acute renal failure main lead to volume overload and pulmonary edema.
Conclusion
In this study, we are able to predict the renal outcomes in patients with rhabdomyolysis on the basis of urine pH. Majority of the patients with urine pH7.0. Urinary alkalization at later stages of AKI is of unproven benefit and may result in salt and water overload and earlier need for renal replacement therapy. Larger multicenter investigation is needed to further define our findings.
Our Findings
1. All patients in this study had elevated serum CPK level.
2. CPK levels varied from 3486 to 57000.
3. There was no direct relationship between serum CPK levels and renal outcomes.
4. Majority of the patients with urine pH<6.5 had AKI.
5. Urine myoglobin varied from 0 to 28,000.
6. There was no direct relationship between urine pH and urine myoglobin levels.
7. There was no relationship between urine myoglobin and GFR
8. Significant relationship between urine pH and AKI was noted.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



