Case Report - (2023) Volume 8, Issue 12
Sarcoidosis: Incidental Finding in Acute Renal Failure, Hypercalcemia, and Elevated Angiotensin Converting Enzyme
2Department of Nephrology and Hypertension, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Glickman Urological & Kidney Institute, Cleveland Clinic, Cleveland Oh, USA
Received Date: Nov 27, 2023 / Accepted Date: Dec 04, 2023 / Published Date: Dec 20, 2023
Abstract
Acute renal failure is a rare presenting feature of sarcoidosis. We report two cases not known to have sarcoidosis, presenting with acute renal failure. Laboratory reports revealed elevated serum creatinine levels as well as hypercalcemia. Renal biopsy showed acute granulomatous interstitial nephritis, non-caseating granulomas, and interstitial fibrosis. Hypercalcemia and renal failure improved to normal levels within two weeks of initiation of steroid therapy. Extensive work up was negative for malignancy. Parathyroid hormone (iPTH) was suppressed, and parathyroid-related peptide (PTHrP) was negative, indicating non-parathyroid origin of hypercalcemia in both cases. Angiotensin converting enzyme(ACE) was elevated in both and returned to normal with steroid therapy. Serum ACE as well as ionized calcium levels may be considered as a biomarker of sarcoid associated hypercalcemia.
Keywords
Sarcoidosis, ACE, AKI, Hypercalcemia
Introduction
Most common cause of hypercalcemia in hospitalized patients is malignancy related, while most common cause of hypercalcemia in the outpatient setting is primary hyperparathyroidism. Relatively uncommon cause of hypercalcemia includes sarcoidosis. Both patients presented with hypercalcemia had calcium levels of 14.3 and 13.8 ng/dL. Both had suppressed iPTH, indicating hypercalcemia of non-parathyroid origin. Both patients had normal parathyroid-related peptide (PTHrP) ruling out malignancy. These patients had acute renal failure, although there was no past history of renal disease. Their past labs showed normal BUN as well as creatinine levels. Both of the patients were treated with Prednisone resulting in normalization of calcium and renal functions. Morphologically, involvement of the kidney in sarcoidosis can occur in three ways: nephrocalcinosis and nephrolithiasis, direct granulomatous involvement of the tubuleintrestitium, or glomerulonephritis [1]. In our patients, ACE levels were elevated on admission and returned to normal after steroid therapy for 2 weeks.
The incidence and prevalence of sarcoid granulomatous nephritis or course of sarcoidosis is not known. Generally, it is a silent finding observed at autopsy in 7-23% of sarcoidosis patients [2]. Sarcoid granulomatous interstitial nephritis is mainly observed at renal biopsies in cases of acute kidney injury, occurring in <1% of sarcoid patients [3]. In some cases, acute kidney injury may progress to chronic kidney disease or even end stage renal disease [4,5]. Generally noted, AKI in sarcoid patients with hypercalcemia was milder than without hypercalcemia. Sarcoidosis is primarily a disease of the lungs and reticuloendothelial system, however the prevalence of renal involvement with sarcoidosis may be under recognized. Pathophysiological mechanisms responsible for renal failure in sarcoidosis include a direct effect of hypercalcemia, dehydration, nephrocalcinosis, and tubule-interstitial disease [1].
Sarcoidosis is a multisystem granulomatous disease effecting people of all races, ethnic groups, and ages. Peak incidents are between the age of 20 and 39 [6]. The incidence among African Americans is three to four times higher than among white Americans and tends to occur later in life [7]. The basic abnormality in sarcoidosis involves formation of immune granulomas in various organs. Lungs and mediastinal lymph nodes are the main organ systems involved, but virtually every organ may be involved [8]. The most frequent sites of sarcoidosis involvement include peripheral lymph nodes, eyes, skin and liver, each being noted in about 10-25% of cases in most series [8].
Sarcoidosis commonly presents with the abnormalities detected on a chest x-ray (8-60%), but the presentation may vary depending on age, sex, race, duration of the disease, and sites of involvement [9]. European population is more likely to present asymptomatically or with erythema nodosum, while symptomatic and multiorgan presentations are more common in African Americans. The initial presentation may include respiratory in 30% cases, constitutional symptoms such as fatigue (27%), weight loss (28%), fever (10-27%), and erythema nodosum (3- 44%) [8]. These symptoms may be mild to severe, depending on duration of the disease and medical treatment rendered. Acute forms of sarcoidosis such as Lofgren syndrome, consists of arthritis, erythema nodosum, bilateral hilar adenopathy (9- 34%), presenting differently in men and women [6]. Although, hypercalcemia occurs in about 11% cases of sarcoidosis [6] clinically significant hypercalcemia is less common [10].
The mechanism of hypercalcemia is not completely understood. The levels of vitamin D and its active metabolite 1, 25-dihydroxyvitamin D play an important role in the maintenance of serum Ca++ levels. Vitamin D is derived either from endogenous production in the skin or by ingestion of Vitamin D supplements. Vitamin D is hydroxalated to 25 hydroxyvitamin D in the liver, which is subsequently converted to 1,25- dihydroxyvitamin D in the renal tubule by enzyme 1, α hydroxylase. This enzyme is tightly regulated by parathyroid hormone (PTH), serum calcium and phosphorus [11]. Granulomas in sarcoidosis express high levels of 1-α hydroxylase, overproduction of 1-α hydroxylase is also responsible for development of hypercalcemia in sarcoid patients. Vitamin D levels are reported to be low leading to physicians prescribing high doses of Vitamin D that may result in hypercalcemia, hypercalciuria, kidney stones and nephrocalcenosis.
In 1939, Harrell and Fisher reported the occurrence of hypercalcemia in 6 of 11 patients with sarcoidosis [12]. On subsequent studies, in 1979, Papapoulos et al and Bell et al were among the first to recognize elevated levels of 1, 25-dihydroxy vitamin D in patients with sarcoidosis [13,14].
Although, acute renal failure as a presenting feature of sarcoidosis has been described, it remains a rare occurrence [9].
Renal pathological changes have been described by Avasthi and Ponce [8,9]:
1. Granulomatous interstitial nephritis
2. Tubular atrophy and intrestitial fibrosis of varying degree
3. Non-caseating granulomas
4. Membranous nephritis has been reported by Ponce, Gujral [19].
Case Studies
2.1 Patient #1
29 year old Caucasian male presented to the hospital with complaints of fever (1010 F), in addition to weakness, nausea, anorexia, weight loss, and increased urination. Patient’s past medical history included hypertension, and non-insulindependent diabetes (NIDDM). Upon admission to the hospital, patient’s blood pressure was stable, but he was noted to be febrile. Patient’s complete blood count (CBC) and comprehensive metabolic panel (CMP) were drawn, CBC was within normal limits. The CMP report showed hypercalcemia, elevated ionized calcium, increased calcium secretion in urine with elevated creatinine demonstrating renal failure. Patient’s ACE levels were almost three times the normal. Renal ultrasound was unremarkable. Subsequently, he underwent lymph node and renal biopsy.
Renal biopsy showed granulomatous interstitial nephritis, moderate tubular atrophy and interstitial fibrosis, as well as moderate arterio- and arteriosclerosis with hyalinosis. as shown in figures 1 (a) to 1 (e) Lymph node biopsy exhibited noncaseating granuloma, consistent with sarcoidosis. While the patient was undergoing work up, he was started on Prednisone that continued for 2 weeks. At the end of two-week, patient’s serum calcium, urine calcium, ironized calcium, and creatinine were back to baseline levels.
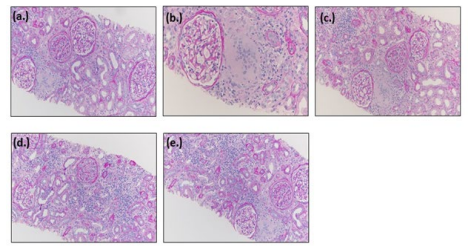
Figure 1: Biopsy Results of Patient #1
2.2 Patient #2
A 62 year old male was admitted to the hospital with fever (100.9 0 F), weakness, anorexia, weight loss, and increased urination with the past medical history of hypertension. Patient’s complete blood count (CBC) and comprehensive metabolic panel (CMP) were drawn. Patient’s CBC was within normal limits, however CMP analysis revealed hypercalcemia, increased ionized calcium, and elevated creatinine suggesting renal failure. Patient’s ace level were twice the normal levels. Alkaline Phosphatase, TSH as week as 25 hydroxy Vit. D were in normal limits. Renal ultrasound was unremarkable. Renal biopsy indicated acute and chronic interstitial nephritis, with severe focal granulation features suggesting sarcoidosis associated syndrome. Lymph node biopsy exhibited non-caseating granuloma, consistent with sarcoidosis. Biopsy results of patient #2 are shown in figure #2 patient was prescribed steroids for two weeks resulting in normalization of serum calcium, urine calcium, ionized calcium, and creatinine.
Table 1 shows details of patients, presentation, history, lab values, as well as imaging and biopsy results.
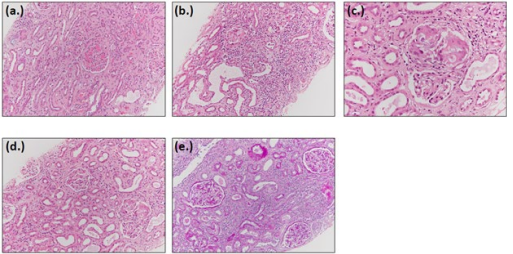
Figure 1: Biopsy Results of Patient #2
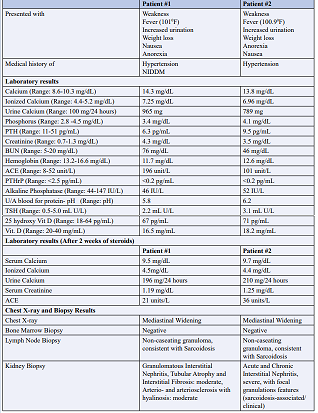
Table 1: Details of patients, presentation, history, lab values, as well as imaging and biopsy results
Discussion
ACE is a glycoprotein enzyme normally secreted by monocytes, macrophages, and pulmonary endothelial cells into the blood stream, and is responsible for physiological reaction of converting Angiotensin I to Angiotensin II, which is important for blood pressure control. Elevated ACE was found in approximately 60% of patients with sarcoidosis [6]. ACE is ectopically produced by epithelial and giant cells in sarcoid granulomas [14,15]. Serum ACE activity can be a biomarker of sarcoid granuloma formation, but it has limited sensitivity through the stages of the disease and in response to corticosteroid treatment [16]. Serum ACE activity may also be increased in a variety of other granulomatous and non-granulomatous conditions, and may be affected by ACE inhibitor medications, which also limits its ability in the diagnosis of sarcoidosis [17].
Increase in ACE has been the most frequently laboratory test in sarcoidosis. The enzyme is supposed to give indication of total body granuloma burden [18,19]. Determination of elevated serum ACE has estimated sensitivity of 57%, specificity 90%, positive predictive value 90%, but negative predictive value 60% [20]. Especially in the first month of acute disease, ACE levels may be in normal range [21]. ACE is thought to be produce by both sarcoid granulomas and lung endothelium [6].
Although the levels of serum angiotensin converting enzyme have been shown to be elevated in sarcoid patients, its sensitivity and specificity as a diagnostic marker may vary, the sensitivity may vary between 41% and 100% and specificity between 83% and 99% [22].
Regardless of these reports, elevated ACE levels may be associated with disease activity and also represent efficient marker of treatment efficacy.
4.Role Of ACE
A recent study preformed on 119 adult sarcoidosis patients, which aimed to establish the role of ACE, showed that elevated ACE levels were associated with high serum levels of ionized calcium [23]. Similarly in a case report by Christina Dana Marginean et al., the author noticed elevated levels of ionized calcium that became normal after treatment with steroids, suggesting indeed that ACE might be a potential therapeutic marker [24].
Calcium metabolism alteration, particularly, was observed in a significant percentage of patients with sarcoidosis. Urinary calcium correlated with clinical status, Diffusing capacity for carbon monoxide (DLCO) and serum CHITOTRIOSIDASE (CTO) activity, suggesting its potential role as biomarker of activity and severity of sarcoidosis.
Hypercalciuria and hypercalcemia were observed in 18.8% and 1.8% of patients respectively [25]. Urinary calcium levels correlated with CHITORIOSIDASE (CTO) activity. Patients with worsening persistent disease showed the highest levels of urinary calcium. [25] DLCO percentages correlated inversely with urinary calcium, suggesting its potential role as biomarker of activity and severity of sarcoidosis.
CTO activity was significantly higher in sarcoidosis patients than in healthy controls. It was higher in both steroid-free and treated groups of patients than healthy controls. In sarcoidosis patients CTO activity and ACE concentration were directly correlated. CTO activity is a reliable biomarker of sarcoidosis. It is correlated with disease activity, severity and multiorgan dissemination.
CTO is produced by sarcoid macrophages and polymorphonuclear neutrophils [26]. It could reflect the number of active granulomas harbored a patient at a given time. CTO expression reduces with steroid therapy, and it was able to detect disease relapse and to identify patients requiring escalation of therapy [25]. Serum CTO levels are almost six times higher in patients with active sarcoidosis than in healthy controls and inactive disease [21]. Levels of CTO activity significantly correlated with disease duration. Regardless of the discordant reports, elevated ACE levels may be associated with disease activity and also represent efficient marker of treatment efficacy [27].
One possible explanation for lower ergocalcitirol levels in sarcoidosis is the enhanced activity of 1-α hydroxylase in sarcoid granulomas. The proportion of calcitriol to ergocalcitriol appears to be higher in sarcoidosis compared to non-sarcoidosis conditions [28]. In one study of 270 patients, 80% had low ergocalcitriol levels but less than 1% had low calcitriol levels. In fact, that study found that 10% of patients had elevated calcitriol levels [29].
Those with elevated calcitriol were more likely to have history of hypercalcemia or hypercalciuria. Higher levels of calcitriol have been associated with more advanced pulmonary sarcoidosis [30]. Papapoulos et al. and Bell et al. are the first to recognize that levels of 1,25- dihydroxyvitamin D are elevated in patients with sarcoidosis [14]. The high levels of 1,25-dihydroxyvitamin D are probable cause of hypercalcemia, but overproduction of bone reabsorbing cytokines and PTH like peptide may also play a role [32].
The renal 1-α hydroxylase is activated by PTH and suppressed by 1,25- dihydroxyvitamin D [11]. The macrophage enzyme is unaffected by one of these factors. Instead, one of its major trophic activators is cytokine interferon gamma [33]. Lack of feedback in response to increased levels of 1,25- dihydroxyvitamin D leads to excess of bone reabsorption in small intestine and in excess of bone reabsorption in patients with sarcoidosis, leading to hypercalcemia.
Hypercalcemia may be a clue to diagnosis of sarcoidosis, especially in cases with no pulmonary symptoms or finding on chest x-ray. Sarcoidosis should be considered in patients with hypercalcemia, low PTH levels, and low calcitriol levels. Long standing hypercalcemia and hypercalciuria can cause nephrocalcinosis and renal failure. Although severe hypercalcemia, associated with renal failure, as was the case in both our patients.
Prednisone 20-40g/day is the drug of choice to reduce endogenous production of 1,25- dihydroxyvitamin D. Administration of steroids can cause a relatively quick decrease of 1,25- dihydroxyvitamin D and serum calcium levels in 3-5 days [34]. Ketoconazole is an alternative for patients where the steroid use is contraindicated. It inhibits the cytochrome p450 linked enzyme system involved in steroid synthesis including 25(OH) D3-1a-hydroxylase [35]. Hydroxychloroquine also causes similar effects and can be used in patients who cannot tolerate ketoconazole or who develops abnormal liver function [36].
Conclusion
Severe symptomatic hypercalcemia is a rare manifestation of sarcoidosis. In both our cases diagnosis was established by lymph node and kidney biopsies after hospital admission for symptoms of hypercalciuria. Acute renal failure, present in both cases, was a result of sarcoidosis with non-caseating granulomas and granulomatous interstitial nephritis. Timely recognition and treatment with steroids abated renal failure and hypercalciuria. ACE and ionized calcium levels elevated on initial hospital presentation in both patients, returned to normal after steroid therapy. ACE may be a significant biomarker of sarcoid induced hypercalciuria. Although, Chitoctriosidase (CTO) levels were not obtained in this study, literature shows its significance in determining severity of sarcoidosis.
Conflict of Interest Statement
None of the authors declare any conflict of interest.
References
1. Awasthi, A., Nada, R., Malhotra, P., Goel, R., & Joshi, K. (2004). Fatal renal failure as the first manifestation of sarcoidosis diagnosed on necropsy in a young man: a case report. Journal of clinical pathology, 57(10), 1101-1103.
2. Longcope, W. T., & PREIMAN, D. G. (1952). A study of sarcoidosis: based on a combined investigation of 160 case’s including 30 autopsies from the Johns Hopkins Hospital and Massachusetts General Hospital. Medicine, 31(1), 1.
3. Javaud, N., Belenfant, X., Stirnemann, J., Laederich, J., Ziol, M., Callard, P., ... & Fain, O. (2007). Renal granulomatoses: a retrospective study of 40 cases and review of the literature. Medicine, 86(3), 170-180.
4. Bagnasco, S. M., Gottipati, S., Kraus, E., Alachkar, N., Montgomery, R. A., Racusen, L. C., & Arend, L. J. (2014). Sarcoidosis in native and transplanted kidneys: incidence, pathologic findings, and clinical course. PLoS One, 9(10), e110778.
5. Mahévas, M., Lescure, F. X., Boffa, J. J., Delastour, V., Belenfant, X., Chapelon, C., ... & Valeyre, D. (2009). Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine, 88(2), 98-106.
6. Iannuzzi, M.C., Rybicki, B.A., & Teirstein, A.S. (2007). Sarcoidosis. New England Journal of Medicine, 357(21), 2153-2108.
7. Rybicki, B. A., Maliarik, M. J., Major, M., Popovich Jr, J., & Iannuzzi, M. C. (1998, September). Epidemiology, demographics, and genetics of sarcoidosis. In Seminars in respiratory infections (Vol. 13, No. 3, pp. 166-173).
8. Nunes, H., Bouvry, D., Soler, P., & Valeyre, D. (2007). Orphanet Journal of Rare. Orphanet Journal of Rare Diseases, 2, 46.
9. Karnchanasorn, R., Sarikonda, M., Aldasouqi, S., & Gossain, V. V. (2010). Severe hypercalcemia and acute renal failure: an unusual presentation of sarcoidosis. Case reports in medicine, 2010.
10. Muther, R. S., McCarron, D. A., & Bennett, W. M. (1981). Renal manifestations of sarcoidosis. Archives of internal medicine, 141(5), 643-645.
11. XHollick, M.F (2007). Vitamin D deficiency. New England Journal of Medicine, 357, 266–281.
12. Harrell, G. T., & Fisher, S. (1939). Blood chemical changes in Boeck’s sarcoid with particular reference to protein, calcium and phosphatase values. The Journal of clinical investigation, 18(6), 687-693.
13. Papapoulos, S. E., Fraher, L. J., Sandler, L. M., Clemens, T. L., Lewin, I. G., & O’Riordan, J. L. H. (1979). 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. The Lancet, 313(8117), 627- 630.
14. Bell, N. H., Stern, P. H., Pantzer, E., Sinha, T. K., & Deluca, H. F. (1979). Evidence that increased circulating 1 α, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. The Journal of clinical investigation, 64(1), 218-225.
15. Kasahara, Y., & Ashihara, Y. (1981). Colorimetry of angiotensin-I converting enzyme activity in serum. Clinical Chemistry, 27(11), 1922-1925.
16. Yasar, Z., Özgül, M. A., Cetinkaya, E., Kargi, A., Gül, Å?., Talay, F., ... & Dincer, H. E. (2016). Angiotensin-converting Enzyme as a Predictor of Extrathoracic Involvement of Sarcoidosis. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases: Official Journal of WASOG, 32(4), 318-324.
17. Brice, E. A., Friedlander, W., Bateman, E. D., & Kirsch, B. E. (1995). Serum angiotensin-converting enzyme activity, concentration, and specific activity in granulomatous interstitial lung disease, tuberculosis, and COPD. Chest, 107(3), 706-710.
18. Ahmadzai, H., Wakefield, D., & Thomas, P. S. (2011). The potential of the immunological markers of sarcoidosis in exhaled breath and peripheral blood as future diagnostic and monitoring techniques. Inflammopharmacology, 19, 55-68.
19. Muthuswamy, P. P., Lopez-Majano, V., Ranginwala, M., & Trainor, W. D. (1987). Serum angiotensin-converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis. Sarcoidosis, 4(2), 142-148.
20. Lynch, J.P., Ling, Ma Y., Koss, M.N., & White, E.S. (2007). Pulmonary Sarcoidosis. Semin Respir Crit Care Med, 28(1), 53-74.
21. Studdy, P. R. (1988). The specificity and sensitivity of serum angiotensin converting enzyme in sarcoidosis and other diseases. Experiences in twelve centers in six different countries. Sarcoidosis. 22. PopeviÄ?, S., Šumarac, Z., JovanoviÄ?, D., BabiÄ?, D., StjepanoviÄ?, M., JoviÄiÄ?, S., ... & MihailoviÄ?-VuÄiniÄ?, V. (2016). Verifying sarcoidosis activity: chitotriosidase versus ace in sarcoidosis–a case-control study. Journal of medical biochemistry, 35(4), 390.
23. Chopra, A., Kalkanis, A., & Judson, M. A. (2016). Biomarkers in sarcoidosis. Expert Review of Clinical Immunology, 12(11), 1191-1208.
24. Sejdic, A., Graudal, N., & Baslund, B. (2018). Clinical and biochemical presentation of sarcoidosis with high and normal serum angiotensin-converting enzyme. Scandinavian Journal of Rheumatology, 47(6), 487-490.
25. MÄ?rginean, C. O., MeliÅ£, L. E., Grigorescu, G., Puiac, C., & Simu, I. (2020). Hypercalcemia, an Important Puzzle Piece in Uncommon Onset Pediatric Sarcoidosis—A Case Report and a Review of the Literature. Frontiers in Pediatrics, 8, 497.
26. Cameli, P., Gonnelli, S., Bargagli, E., d’Alessandro, M., Bergantini, L., Favetta, V., ... & Caffarelli, C. (2020). The role of urinary calcium and chitotriosidase in a cohort of chronic sarcoidosis patients. Respiration, 99(3), 207-212.
27. Harlander, M., Salobir, B., ZupanÄiÄ, M., Dolenšek, M., Vodovnik, T. B., & TerÄelj, M. (2014). Serial chitotriosidase measurements in sarcoidosis–two to five year follow-up study. Respiratory medicine, 108(5), 775-782.
28. Baughman, R. P., Ploysongsang, Y., Roberts, R. D., & Srivastava, L. (1983). Effects of sarcoid and steroids on angiotensin-converting enzyme. American Review of Respiratory Disease, 128(4), 631-633.
29. Baughman, R. P., & Lower, E. E. (2014). Goldilocks, vitamin D and sarcoidosis. Arthritis research & therapy, 16, 1-2.
30. Baughman, R. P., Janovcik, J., Ray, M., Sweiss, N., & Lower, E. E. (2013). Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG, 30(2), 113-120.
31. Kavathia, D., Buckley, J. D., Rao, D., Rybicki, B., & Burke, R. (2010). Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respiratory medicine, 104(4), 564-570.
32. Zeimer, H. J., Greenaway, T. M., Slavin, J., Hards, D. K., Zhou, H., Doery, J. C., ... & Grill, V. (1998). Parathyroidhormone-related protein in sarcoidosis. The American journal of pathology, 152(1), 17.
33. Monkawa, T., Yoshida, T., Hayashi, M., & Saruta, T. (2000). Identification of 25-hydroxyvitamin D3 1α-hydroxylase gene expression in macrophages. Kidney international, 58(2), 559- 568.
34. Sharma, O. P. (2000). Hypercalcemia in granulomatous disorders: a clinical review. Current opinion in pulmonary medicine, 6(5), 442-447.
35. ADAMS, J. S., SHARMA, O. P., DIZ, M. M., & ENDRES, D. B. (1990). Ketoconazole decreases the serum 1, 25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. The Journal of Clinical Endocrinology & Metabolism, 70(4), 1090-1095.
36.Adams, J. S., Diz, M. M., & Sharma, O. P. (1989). Effective reduction in the serum 1, 25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia with short-course chloroquine therapy. Annals of internal medicine, 111(5), 437-438.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



