Case Report - (2023) Volume 8, Issue 7
Relapse of Membranous Glomerulonephritis with Crescentic Transformation Following Covid-19 Vaccination: A Case Report
2Brighton and Sussex Medical School, Brighton, Sussex Kidney Unit, University Hospitals Sussex NHS Foundation Trust, Brighton, United Kingdom., UK
Received Date: Jul 07, 2023 / Accepted Date: Jul 22, 2023 / Published Date: Jul 28, 2023
Abstract
Crescentic transformation in membranous glomerulonephritis is an uncommon form of the disease, which usually presents with heavy proteinuria, haematuria and renal dysfunction. Several vaccines have been associated with kidney-related issues, including reports of de novo or recurrent glomerular disease associated with Covid-19 vaccination. We describe a case of relapse of primary membranous nephropathy with crescentic transformation in temporal association with the first dose of the Oxford-AstraZeneca Covid-19 vaccine.
Keywords
Membranous glomerulonephritis, Crescentic transformation, Covid-19, Case report
Abbreviations
ACE: Angiotensin-Converting Enzyme; ANCA: Antineutrophilic Cytoplasmic Antibody; anti-GBM: anti-Glomerular Basement Membrane Antibody; COVID-19: Coronavirus Disease 2019; ESRD: End-Stage Renal Disease; IgA: Immunoglobulin A; GFR: Glomerular Filtration Rate; g/l: Gram Per Litre; MRI: Magnetic Resonance Imaging; MGN: Membranous Glomerulonephritis; mRNA: Messenger Ribonucleic Acid; mg/mmol: Milligrams Per Millimole; mmol/l: Millimoles Per Litre; PL2R: Phospholipase A2 Receptor; RU/ml: Relative Units Per Millilitre; UPCR: Urine Protein: Creatinine Ratio.
Introduction
Membranous glomerulonephritis (MGN) is a glomerular disease that is the most common cause of nephrotic syndrome in adults. In the case of primary membranous nephropathy, which accounts for 80% of membranous cases, no underlying cause is detected. The remaining 20% of cases constitute secondary membranous nephropathy associated with medications, systemic lupus erythematosus, hepatitis virus infection or malignancies. The incidence of crescent transformation in MGN is an uncommon form of the disease [1,2]. Crescents may be detected when MGN first presents and called crescentic membranous glomerulonephritis (GN) or develop later, known as crescentic transformation. It usually presents with heavy proteinuria, haematuria and glomerular filtration rate (GFR) decline [3].
Several past vaccines have been associated with kidneyrelated issues [4]. Certain vaccines, such as those for influenza, pneumococcal, and hepatitis B, have been linked to acute kidney injury and various glomerular diseases following immunisation.
We describe a case where a relapse of MGN with crescentic transformation occurred after the first dose of the OxfordAstraZeneca Covid-19 vaccine, where there seems to be a temporal association between the two events.
Case Presentation
A man in his late 60s was diagnosed with membranous nephropathy in 2009 and received treatment with a cyclophosphamide-based modified Ponticelli regime, achieving sustained partial remission. When he was transferred to the Sussex Kidney Unit in 2014, he was found to have preserved renal function with modest proteinuria (urine protein:creatinine ratio (UPCR)<100 mg/mmol). However, despite the presence of Phospholipase A2 receptor (PLA2R) antibodies, he was not on maintenance immunosuppression and had not previously received annual influenza vaccination.
Following receiving the first dose of the Oxford-AstraZeneca viral vector-based vaccine in February 2021, the patient’s nephrotic syndrome relapsed. He presented with significant lower limb swelling, nephrotic-range proteinuria and a 20% increase in serum creatinine. Peak serum creatinine reached 200 mmol/l, and peak UPCR was 1,141 mg/mmol. Serological tests at the time showed positive PLA2R antibody 98 RU/ml and negative anti-neutrophil cytoplasm antibody (ANCA) and antiglomerular basement membrane antibody (anti-GBM).
Despite being PLA2R positive, the subsequent rise in creatinine prompted a percutaneous renal biopsy which revealed membranous nephropathy with two cellular crescents and segmental sclerosis, interstitial fibrosis, and acute tubular injury. In addition, electron microscopy showed frequent subepithelial electron-dense deposits, but no subendothelial or mesangial deposits (Figure 1).
Further, work-up to exclude secondary causes of membranous nephropathy included viral serology screening and screening for lymphoproliferative neoplasms, which were negative. In addition, ultrasound and dedicated MRI imaging of the renal vessels excluded renal venous thrombosis.
The patient agreed to receive treatment with rituximab and a slowly weaning regimen of oral corticosteroids. His serum creatinine and proteinuria levels had fallen to return to prerelapse levels by nine months and 18 months post-treatment, respectively. He was additionally anticoagulated on warfarin (throughout the period when serum albumin was below 25 g/l), commenced statin therapy and remains on an angiotensinconverting enzyme (ACE) inhibitor. The patient declined the second dose of the vaccination.
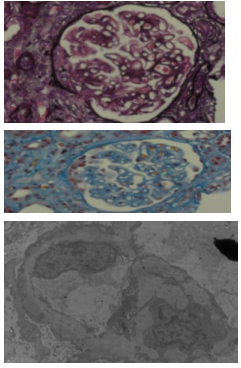
Figure 1: Renal biopsy: top: Silver stain, middle: MSB (Trichrome) stain, lower: Electron microscopy
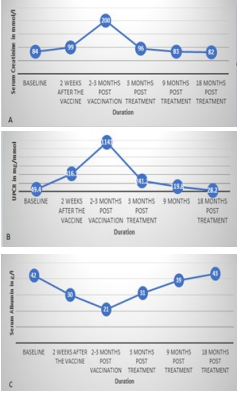
Figure 2: A) Serum Creatinine, B) UPCR, C) Serum albumin before and after treatment.
Discussion
Worldwide mass vaccination programmes have sought to deliver herd protection against Covid-19 infection, and hundreds of millions of Oxford-AstraZeneca Covid-19 vaccine doses have been purchased and administered [5]. However, given the volume of mass vaccinations, rarely observed presentations might be recorded by chance or reflect a causal link with vaccination. It is important to emphasise that the nature of case reports is anecdotal and does not provide definitive evidence of a causal relationship between the vaccine and the reported renal condition. However, on review of the published literature, the authors have identified a number of other published reports that described cases with a temporal association between Covid-19 vaccination and the development of crescentic glomerulonephritis, including cases that were antibody negative or associated with positive ANCA [6-9], anti-GBM [10], lupus nephritis [11], PLA2R antibodies [12] or IgA nephropathy [13,14]. In addition, other case reports have described de novo and recurrent glomerular disease, including minimal change disease [15-22], membranous nephropathy [23,24] and IgA nephropathy [25-28] following Covid-19 vaccination, many with presentation one to two weeks following receiving the vaccine. To the best of our knowledge, our case presents the first temporal association of viral-vector-based Covid-19 vaccination with a relapse of membranous nephropathy with crescentic transformation.
Crescentic membranous glomerulonephritis is an infrequent presentation. The cause of this crescent transformation is unknown. It can be associated with positive ANCA, anti-GBM, anti-PLA2R antibodies or negative serology. In this case, only the PLA2R antibody was present before and after vaccination. It is unclear if the positivity of ANCA or anti-GBM represents the co-incidence of two separate diseases or if they have related pathogenicity. Related pathogenicity may be explained by the damage that subepithelial deposits cause to the glomerular basement membrane leading to the formation of anti-GBM. The opposite can happen as anti-GBM can damage the epitopes leading to subepithelial immune complex deposition causing membranous nephropathy and facilitating subepithelial deposits [1]. This case of relapsed MGN with crescents, rapidly rising serum creatinine levels, and PLA2R antibodies has been managed with oral steroids and the anti-CD20- Rituximab.
About 70% of patients with primary disease have positive PLA2R antibodies. In this case, despite the positive PLA2R antibodies, which point to primary membranous nephropathy, renal biopsy was highly indicated due to the worsening kidney function and the time of relapse post-Covid-19 vaccine. In the case of PLA2R-positive antibodies, if there is impaired kidney function, a kidney biopsy is recommended to rule out specific diagnoses like crescentic membranous and evaluate the degree of interstitial fibrosis and tubular atrophy as this can direct the management [3]. This disease differs from classical membranous nephropathy as its course is more aggressive, which can progress to end-stage renal disease (ESRD). Crescentic membranous nephropathy should be managed the same as rapidly progressive glomerulonephritis with steroids plus cyclophosphamide or Rituximab [1].
Because of the scarce available evidence, we had difficulty counselling the patient on whether further doses of the vaccine would provide greater benefit than risk and whether having a different type of the vaccine, may it be a messenger ribonucleic acid (mRNA) vaccine, protein subunit or a different viral vector-based vaccine, would lead to a different outcome or decrease the risk of relapse.
A systematic review by Wu et al [29] in 2021 of de novo and relapsed renal pathologies associated with Covid-19 vaccination examined 48 cases from 36 published articles and found that minimal change disease was the most frequent pathology observed with 19 cases in their review, ahead of IgA nephropathy with 14 cases, and vasculitis with 10 cases reported. The review also concluded that the benefits of Covid-19 vaccination in terms of protection are much greater than the risks involved. Nevertheless, they recommended performing an early biopsy for patients who exhibit symptoms of new-onset kidney disease following vaccination and closely monitoring symptoms in those with possible relapse.
Collation of data from large groups of vaccine-associated cases of relapsed or de novo glomerular disease may assist in understanding the risks, mechanisms of and treatment of glomerular presentations associated with Covid-19 vaccination.
Conclusion
Glomerulonephritis was one of the reported complications after Covid-19 vaccination. Mass vaccination of different populations led to the increased observation of rare complications. This case showed that patients with membranous GN could have a risk of relapse and crescentic transformation. Follow up of those patients is recommended after exposure to the vaccine to detect any relapse. Early renal biopsy and management is recommended if deterioration of the kidney function or the degree of proteinuria is found. In the case of positive PLA2R antibodies, worsening kidney function gives a strong indication for renal biopsy. Having a registry for those cases of GN complicating Covid-19 vaccines is required to assess the spectrum of the condition.
Acknowledgement
Not applicable.
Author Contributions
SB made major contributions in writing the manuscript. FG summarised the laboratory findings and prepared the figures, shared in drafting the case report and substantially revised the manuscript. All authors read and approved the final manuscript.
Funding Sources
None.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Consent for Publication
Informed consent was obtained from the patient for publication of this case report.
Competing Interests
The authors declare that they have no competing interests.
References
1. Nikolopoulou, A., Huang-Doran, I., McAdoo, S.P., Griffith, M.E., Cook, H.T., & Pusey, C.D. (2019). Membranous glomerulonephritis with crescents. Kidney International Reports, 4(11), 1577-1584.
2. Rodriguez, E.F., Nasr, S.H., Larsen, C.P., Sethi, S., Fidler, M.E., & Cornell, L.D. (2014). Membranous nephropathy with crescents: a series of 19 cases. American Journal of Kidney Diseases, 64(1), 66-73.
3. Ronco, P., Beck, L., Debiec, H., Fervenza, F.C., Hou, F.F., Jha, V., & Wetzels, J. (2021). Membranous nephropathy. Nature reviews Disease primers, 7(1), 69.
4. Patel, C., & Shah, H.H. (2019). Vaccine-associated kidney diseases: A narrative review of the literature. Saudi Journal of Kidney Diseases and Transplantation, 30(5), 1002-1009.
5. “One year anniversary of UK deploying Oxford-AstraZeneca vaccine”. gov.uk (Press release). Archived from the original on 4 January 2022. Retrieved 4 January 2022.
6. Dube, G.K., Benvenuto, L.J., & Batal, I. (2021). Antineutrophil cytoplasmic autoantibody–associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney International Reports, 6(12), 3087-3089.
7. Shakoor, M.T., Birkenbach, M.P., & Lynch, M. (2021). ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. American Journal of Kidney Diseases, 78(4), 611-613.
8. Hakroush, S., & Tampe, B. (2021). Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Frontiers in Immunology, 12, 762006.
9. Sekar, A., Campbell, R., Tabbara, J., & Rastogi, P. (2021). ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney international, 100(2), 473-474.
10. Sacker, A., Kung, V., & Andeen, N. (2021). Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney international, 100(2), 471-472.
11. Tuschen, K., Bräsen, J.H., Schmitz, J., Vischedyk, M., & Weidemann, A. (2021). Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney International, 100(4), 941-944.
12. Aydın, M.F., Yıldız, A., Oruç, A., Sezen, M., Dilek, K., Güllülü, M., & Ersoy, A. (2021). Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney international, 100(2), 464-465.
13. Sugita, K., Kaneko, S., Hisada, R., Harano, M., Anno, E., Hagiwara, S., & Tsukamoto, Y. (2022). Development of IgA vasculitis with severe glomerulonephritis after COVID-19 vaccination: a case report and literature review. CEN Case Reports, 11(4), 436-441.
14. Ran, E., Wang, M., Wang, Y., Liu, R., Yi, Y., & Liu, Y. (2022). New-onset crescent IgA nephropathy following the CoronaVac vaccine: A case report. Medicine, 101(33).
15. Lebedev, L., Sapojnikov, M., Wechsler, A., Varadi-Levi, R., Zamir, D., Tobar, A., & Yagil, Y. (2021). Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. American Journal of Kidney Diseases, 78(1), 142-145.
16. Maas, R.J., Gianotten, S., & van der Meijden, W.A. (2021). An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. American Journal of Kidney Diseases, 78(2), 312.
17. D’Agati, V.D., Kudose, S., Bomback, A.S., Adamidis, A., & Tartini, A. (2021). Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney international, 100(2), 461-463.
18. Kervella, D., Jacquemont, L., Chapelet-Debout, A., Deltombe, C., & Ville, S. (2021). Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney International, 100(2), 457-458. 19. Thappy, S., Thalappil, S.R., Abbarh, S., Al-Mashdali, A., Akhtar, M., & Alkadi, M.M. (2021). Minimal change disease following the Moderna COVID-19 vaccine: first case report. BMC nephrology, 22, 1-4.
20. Komaba, H., Wada, T., & Fukagawa, M. (2021). Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. American Journal of Kidney Diseases, 78(3), 469-470.
21. Morlidge, C., El-Kateb, S., Jeevaratnam, P., & Thompson, B. (2021). Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney International, 100(2), 459.
22. Weijers, J., Alvarez, C., & Hermans, M.M. (2021). Post-vaccinal minimal change disease. Kidney International, 100(2), 459-461.
23. Gueguen, L., Loheac, C., Saidani, N., & Khatchatourian, L. (2021). Membranous nephropathy following anti– COVID-19 mRNA vaccination. Kidney International, 100(5), 1140-1141.
24. Paxton, L., McMahon, L., & Wong, L. (2022). De novo PLA2R positive membranous nephropathy following BNT162b2 mRNA COVIDâ?19 vaccine. Internal Medicine Journal.
25. Nihei, Y., Kishi, M., Suzuki, H., Koizumi, A., Yoshida, M., Hamaguchi, S., & Suzuki, Y. (2022). IgA nephropathy with gross hematuria following COVID-19 mRNA vaccination. Internal Medicine, 61(7), 1033-1037.
26. Nakatani, S., Mori, K., Morioka, F., Hirata, C., Tsuda, A., Uedono, H., & Emoto, M. (2022). New-onset kidney biopsy-proven IgA vasculitis after receiving mRNA-1273 COVID-19 vaccine: case report. CEN Case Reports, 11(3), 358-362.
27. Rahim, S.E.G., Lin, J.T., & Wang, J.C. (2021). A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney international, 100(1), 238.
28. Negrea, L., & Rovin, B.H. (2021). Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney International, 99(6), 1487. 29. Wu, H.H., Kalra, P.A., & Chinnadurai, R. (2021). New-onset and relapsed kidney histopathology following COVID-19 vaccination: a systematic review. Vaccines, 9(11), 1252.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



