Review Article - (2024) Volume 5, Issue 4
Pulse Modulated Neural Nets with Adaptive Temporal Windows for Enhanced Video Perception
Received Date: Oct 24, 2024 / Accepted Date: Nov 04, 2024 / Published Date: Dec 10, 2024
Copyright: ©2024 Greg Passmore. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Passmore, G. (2024). Pulse Modulated Neural Nets with Adaptive Temporal Windows for Enhanced Video Perception. Adv Mach Lear Art Inte, 5(4), 01-26.
Abstract
A key challenge in modern artificial intelligence is the limited awareness of temporal dynamics. This is especially evident in AI's struggles with video animation and interpreting sequential frames or time-dependent data. This paper presents a new method for modulating neuron spikes to synchronize with incoming frames, addressing the temporal limitations of voltage AI models.
This approach involves a specialized class of neuron, similar to Wolfgang Maass's liquid state machine concept. However, our model differs significantly in its synchronization strategy. Neurons are synchronized with frame import, incorporating a flexible time window that enables spike modulation ranging from 1 to 1024 spikes per frame. This adaptability is crucial for accurately processing and interpreting time-based data.
The main focus of this paper is to explore the operational principles of these specialized neurons. While the wiring and architectural design of these neurons will be discussed in a future article, this paper aims to establish the foundational understanding of how these neurons function individually and in response to temporal data inputs.
By synchronizing neuron spikes with frame inputs and allowing for variable spike modulation, this method aims to enhance AI's ability to handle time-based data. The proposed neuron model represents a step towards more advanced and temporally aware AI systems, potentially unlocking new capabilities in video animation and other time-sensitive applications.
Biomimicry
In this study, we highlight the neuron as the fundamental unit of granularity in artificial neural networks, aiming to create an advanced neuron model that excels in time-based processing. The rationale behind this approach is rooted in biomimicry, the practice of emulating biological systems and processes in engineering and technology. By closely studying human neuron types and behaviors, we aim to extract valuable insights that can inform the design of artificial neurons with enhanced capabilities for handling temporal information.
The value of biomimicry in this context lies in its potential to bridge the gap between the complex, dynamic functionality of biological neurons and the comparatively static nature of traditional artificial neurons. While classic artificial neuron models are effective in various applications, they often struggle in tasks requiring sophisticated time-based analysis. These conventional models lack the inherent mechanisms for temporal integration and dynamic adaptation found in biological neurons, making them less suitable for processing sequential or time-dependent data, such as video streams.
Biological neurons possess remarkable abilities for encoding, transmitting, and processing temporal information. This includes generating action potentials, synaptic plasticity, and intricate network interactions, all contributing to their ability to adapt to changing stimuli over time. In contrast, classic artificial neurons typically employ static activation functions and do not inherently consider the temporal aspects of input data. As a result, they struggle to effectively capture the nuances and dynamics inherent in time-based processes like video analysis.
Our objective is to leverage the principles of bio mimicry to design a novel neuron model that overcomes these limitations. By incorporating features inspired by the adaptive and temporal processing capabilities of biological neurons, we aim to create artificial neurons that are more proficient in handling timedependent data. This entails integrating mechanisms for temporal sequencing, dynamic threshold adjustment, and synaptic plasticity, which are vital for accurately interpreting and responding to video content.
The development of such a neuron model has the potential to significantly enhance the performance of artificial neural networks in video processing and other time-critical applications. By closely resembling the functionality of biological neurons, these improved artificial neurons could offer greater accuracy, efficiency, and adaptability in tasks involving temporal data analysis.
Neuronal Diversity
The human brain, a marvel of complexity and functionality, comprises approximately 10,000 distinct types of neurons, each with its unique role and characteristics. Broadly speaking, these diverse neuron types can be classified into three main categories: motor neurons, sensory neurons, and interneurons. This categorization is based on the primary functions these neurons serve within the neural network of the brain and body.
Motor neurons play a vital role in transmitting motor information from the brain and spinal cord to the muscles and glands. These neurons serve as the executors of the nervous system, translating neural signals into physical actions and responses. Whether it involves the precise movement of fingers or the regulation of glandular secretions, motor neurons are essential in transforming neural instructions into tangible outputs.
In contrast, sensory neurons are responsible for relaying sensory information from various parts of the body to the brain and spinal cord. These neurons act as the sensory input channels of the nervous system, converting external stimuli such as light, sound, touch, and temperature into electrical signals that can be processed by the brain. Sensory neurons enable the perception of the environment and the body's interaction with it, forming the foundation of our senses.
Interneurons act as intermediaries within the nervous system, transmitting information between different types of neurons. Predominantly found in the brain and spinal cord, interneurons are vital for the integration and processing of information. They form intricate networks that facilitate communication between sensory and motor neurons, as well as between neurons within the brain. Interneurons play a crucial role in reflex actions, higher cognitive functions, and the modulation of sensory and motor pathways.
The diversity of neurons extends beyond their functional classification; neurons also exhibit various shapes and sizes, each tailored to their specific roles. This morphological diversity is evident in the distinct structures of neurons, ranging from the elongated axons of motor neurons that can extend to distant muscles to the intricate dendritic trees of interneurons that enable extensive synaptic connections.
Neuronal Firing
Depolarization in neurons refers to a change in the neuron's membrane potential that renders it less negative relative to the outside. More technically, it signifies a shift in the membrane potential towards a less negative value. This process plays a pivotal role in the generation and propagation of electrical signals, known as action potentials, within neurons.
To comprehend depolarization, it is crucial to first understand the resting membrane potential of neurons. Neurons at rest maintain a negative membrane potential, typically around -70 millivolts (mV), due to differences in ion concentrations inside and outside the cell and the selective permeability of the neuron's membrane to these ions. The sodium-potassium pump primarily establishes this resting potential by actively transporting sodium ions (Naâº) out of the neuron and potassium ions (Kâº) into the neuron.
During depolarization, there is a temporary change in the neuron's membrane permeability, enabling Na⺠ions to enter the cell. This influx of positively charged sodium ions reduces the charge difference across the membrane, causing the membrane potential to become more positive (less negative). If this depolarization reaches a certain threshold, usually around -55 mV, it triggers an action potential.
An action potential is a rapid, transient change in membrane potential that travels along the neuron's axon and is used for transmitting signals over long distances within the nervous system. After depolarization, the neuron undergoes repolarization, where the membrane potential returns to its resting negative value, and hyperpolarization, a state where the membrane potential becomes even more negative than the resting potential. These processes involve the outflow of K⺠ions and the closure of Na⺠ion channels.
In summary, depolarization in neurons is an important step in the process of neural communication, enabling the generation of action potentials that facilitate the transmission of information throughout the nervous system.
Hyperpolarization in neurons refers to a change in the neuron's membrane potential that renders it more negative than the resting membrane potential. This process occurs after an action potential and is an essential aspect of the neuron's return to its resting state.
To delve deeper into this phenomenon, let's consider the sequence of events in an action potential. After a neuron fires an action potential, it undergoes a phase known as repolarization, where the membrane potential returns toward the resting level. This repolarization is primarily due to the closure of sodium ion (Naâº) channels and the opening of potassium ion (Kâº) channels. As a result, K⺠ions flow out of the neuron, driven by both their concentration gradient and the electrical gradient.
Hyperpolarization follows repolarization and is characterized by the membrane potential becoming even more negative than the resting potential. This is mainly because the K⺠channels remain open slightly longer than necessary to reach the resting potential. The continued efflux of K⺠ions out of the neuron increases the negative charge inside the cell, thus causing hyperpolarization.
During hyperpolarization, the neuron is less responsive to stimuli compared to its resting state. This period is known as the refractory period, during which it is more difficult or impossible for the neuron to fire another action potential. The refractory period ensures that action potentials travel in one direction along an axon and that they are discrete events rather than continuous signals.
Once the refractory period is over, the neuron's membrane potential returns to its resting state. This is achieved through the action of the sodium potassium pump (Naâº/Kâº-ATPase), which actively transports Na⺠ions out of the neuron and K⺠ions into the neuron, restoring the original ion concentration gradients. The neuron is then ready to respond to new stimuli and potentially fire another action potential.
In summary, hyperpolarization is a phase following an action potential in which the neuron's membrane potential becomes more negative than the resting potential. This phase contributes to setting the refracttory period, thereby regulating the timing and directionality of action potentials in neuronal signaling.
The duration of the various states associated with an action potential in neurons — depolarization, repolarization, and hyperpolarization — varies depending on the type of neuron and the conditions under which it is operating. However, to provide a general idea, we can discuss typical timeframes for these phases in mammalian neurons under normal physiological conditions.
Depolarization: The depolarization phase is rapid, typically lasting about 1 millisecond (MS). During this phase, voltage-gated sodium channels open, allowing an influx of sodium ions into the neuron, which causes the membrane potential to become more positive.
Repolarization: Following depolarization, repolarization also occurs quickly, usually within about 1 MS. In this phase, the sodium channels start to inactivate, and voltage-gated potassium channels open, allowing potassium ions to flow out of the neuron. This outflow of positively charged ions restores the membrane potential toward its resting negative value.
Hyperpolarization: The hyperpolarization phase is slightly longer, typically lasting a few milliseconds. During this phase, the potassium channels remain open longer than needed just to reach the resting potential, resulting in the membrane potential becoming more negative than the resting level. This is the period during which the neuron is in the refractory state.
Refractory Periods: The refractory period consists of two parts:
• Absolute Refractory Period: This period lasts approximately 1-2 MS during the depolarization and most of the repolarization phase. During this time, the neuron cannot fire another action potential regardless of the strength of the stimulus.
• Relative Refractory Period: This period occurs during the hyperpolarization phase and may last several milliseconds. During this time, it is possible to trigger another action potential, but only with a stronger than normal stimulus.
It's important to note that these durations are approximate and can vary. Factors such as neuron type, temperature, and the specific physiological conditions of the neuron can affect the exact timing of these phases. For instance, in some types of neurons or under different physiological conditions, the duration of these phases can be longer or shorter.
Visual Processing Pipeline
Visual processing is a complex process that involves multiple steps and various neural cell types at each stage. The process begins with the capture of light by the eyes and ends with the perception of images in the brain. Here's an overview of the steps in visual processing and the neural cells involved:
Light Reception in the Retina: Photoreceptors (Rods and Cones): Light first enters the eye and is captured by photoreceptor cells in the retina. Rods are responsible for low-light (scotopic) vision and do not discern color, while cones are responsible for color (photopic) vision and function best in well-lit conditions.
Photoreceptors, comprising rods and cones, are specialized neurons located in the retina, responsible for the initial transduction of light into neural signals. These cells are the primary interface between the visual world and the nervous system, playing a pivotal role in the process of vision. The unique characteristics, functional properties, and types of photoreceptors are essential to understanding the complexity of visual processing.
Photoreceptors are uniquely adapted to capture light and convert it into electrochemical signals. This process begins when light photons strike photopigments within the photoreceptors, triggering a cascade of biochemical reactions that ultimately lead to changes in membrane potential. These changes are then transmitted to downstream neurons in the retina, initiating the complex process of visual perception.
Rods
• Function and Specialization: Rods are highly sensitive to light and are responsible for vision under low-light conditions, a function known as scotopic vision. They are more numerous than cones and are predominantly located in the peripheral regions of the retina.
• Photopigment: The photopigment in rods is rhodopsin, which is particularly sensitive to dim light. Rhodopsin's high sensitivity enables rods to detect low levels of illumination, making them essential for night vision.
• Signal Transduction: Upon absorption of light, rhodopsin undergoes a conformational change, activating a G-protein-coupled receptor pathway that leads to the closure of sodium channels, hyperpolarizing the rod cell.
• Spatial Summation: Rods are often connected to the same bipolar cells, a feature that allows for spatial summation. This convergence enhances light sensitivity but reduces spatial resolution, making rod-mediated vision less detailed but more sensitive to low light levels.
Cones
• Function and Specialization: Cones are responsible for highacuity, color vision under well-lit conditions, known as photopic vision. They are less sensitive to light than rods but provide the ability to discern fine details and color.
• Photopigments: Cones contain one of three different photopigments, each sensitive to different wavelengths of light (short, medium, or long wavelengths), corresponding to blue, green, and red light, respectively. This trichromatic system forms the basis of color vision in humans.
• Signal Transduction: Similar to rods, cones transduce light into neural signals through a G-protein-coupled receptor pathway. However, the photopigments in cones have lower sensitivity to light, requiring brighter illumination for activation.
• Distribution and Acuity: Cones are densely packed in the fovea, the central region of the retina, providing high spatial resolution and visual acuity. This concentration of cones enables detailed and color vision, essential for tasks like reading and recognizing faces.
The distinct characteristics of rods and cones, including their specific photopigments and light sensitivity, as well as their distribution and connectivity patterns in the retina, demonstrate the intricate design of the visual system. The specialized functions of these photoreceptors enable humans to perceive a wide range of lighting conditions and colors, forming the foundation of our rich and dynamic visual experience. The study of rods and cones continues to be a crucial area in vision research, providing insights into the fundamental mechanisms of light perception and potential therapies for retinal diseases.
Preliminary Processing in the Retina
Horizontal Cells: These cells integrate signals from multiple photoreceptors and modulate the input to bipolar cells, contributing to contrast enhancement and spatial processing. Horizontal cells are a distinct type of neuron found in the retina, playing a critical role in the processing of visual information. These cells are interneurons that integrate and modulate signals between photoreceptors (rods and cones) and bipolar cells. Horizontal cells are known for their distinctive features and contribution to several key aspects of visual processing, including contrast enhancement and color perception.
Morphological Characteristics:
• Horizontal cells are characterized by their wide and laterally extended dendritic and axonal arbors, which allow them to connect with numerous photoreceptors and bipolar cells across a broad area of the retina.
• There are typically two main types of horizontal cells based on their morphology and connectivity: Type I (or H1), which connects primarily with cones, and Type II (or H2), which connects with both rods and cones. Some species also have a Type III horizontal cell.
Function in Lateral Inhibition
• One of the primary functions of horizontal cells is to mediate lateral inhibition, a process that sharpens and enhances visual information. By inhibiting the activity of neighboring photoreceptors and bipolar cells, horizontal cells increase the contrast and definition of the visual image.
• Lateral inhibition is crucial for edge detection and the perception of spatial detail. It allows the visual system to detect borders and transitions between light and dark areas more effectively.
Role in Color Perception
• Horizontal cells are also involved in color processing, particularly in the antagonistic center-surround organization of photoreceptor receptive fields. This organization is important for color contrast and the ability to distinguish colors in complex visual scenes.
• By integrating signals from different types of cones, horizontal cells contribute to the processing of color information and help in minimizing chromatic aberration.
Signal Integration and Feedback
• Horizontal cells receive input from photoreceptors and provide feedback to both photoreceptors and bipolar cells. This feedback mechanism modulates the photoreceptor response to light and influences the bipolar cell output, impacting the overall visual processing in the retina.
• The extensive network of horizontal cell connections enables them to integrate visual information over a large area, contributing to the uniformity and consistency of the visual field.
Unusual Features and Adaptation
• Horizontal cells exhibit unique electrophysiological properties, including graded potentials rather than action potentials, allowing them to modulate signal strength continuously. • They play a role in the adaptation of the retina to different lighting conditions. By modulating the sensitivity of photoreceptors and bipolar cells, horizontal cells help the retina adjust to varying levels of illumination.
Bipolar Cells
Bipolar cells receive input from photoreceptors and horizontal cells, and they relay signals to retinal ganglion cells. They are crucial in processing visual information before it is sent to the brain.
Bipolar cells are integral components of the retinal circuitry, playing a crucial role in the processing of visual information within the eye. Situated between the photoreceptors (rods and cones) and the retinal ganglion cells (RGCs), bipolar cells act as intermediaries, conveying and modulating signals from the photoreceptors to the RGCs. The unique characteristics and diverse subtypes of bipolar cells contribute significantly to the initial stages of visual processing.
Bipolar cells are named for their distinct structure, featuring two distinct processes: one dendritic process that receives inputs from photoreceptors and one axonal process that forms synapses with RGCs and, in some cases, amacrine cells. This bipolar structure allows them to effectively transmit and transform visual information from the outer to the inner retina.
One of the key functions of bipolar cells is to process and convey information about light intensity and color from photoreceptors to RGCs. They play a vital role in establishing the basis for contrast sensitivity, color perception, and spatial resolution in vision. Bipolar cells achieve this through various mechanisms:
• Direct and Indirect Pathways: Some bipolar cells establish direct synaptic connections with photoreceptors, providing a pathway for rapid and precise transmission of visual information. Other bipolar cells are connected indirectly through horizontal cells, which modulate the input from photo receptors, contributing to contrast enhancement and center-surround receptive field organization.
• ON and OFF Pathways: Bipolar cells are broadly classified into two types based on their response to light: ON bipolar cells and OFF bipolar cells. ON bipolar cells depolarize (become more excited) in response to an increase in light intensity in their receptive field center, while OFF bipolar cells depolarize in response to a decrease in light intensity. This distinction is crucial for encoding changes in light intensity and contributes to the detection of luminance contrast.
• Subtypes and Specialization: There are multiple subtypes of bipolar cells, each specialized for different aspects of visual processing. Some bipolar cells are connected predominantly to cones and are involved in high-acuity, color vision. Others are connected to rods and are responsible for processing low-light (scotopic) vision. The specific connections and properties of these subtypes enable the retina to process a wide range of visual information under varying lighting conditions.
• Center-Surround Receptive Fields: Similar to RGCs, bipolar cells contribute to the formation of center-surround receptive fields, a fundamental property of retinal processing. This organization enhances the retina's ability to detect edges and contrasts in the visual scene, essential for spatial resolution and pattern recognition.
Bipolar cells are not mere passive conduits in the visual pathway; they play an active and dynamic role in processing visual information. Their diverse subtypes and specialized functions are instrumental in encoding various aspects of the visual scene, such as light intensity, color, and contrast. The complexity and adaptability of bipolar cells underscore the sophistication of the retinal circuitry.
Amacrine Cells
These interneurons interact with bipolar cells and retinal ganglion cells, playing a role in complex visual processing such as motion detection and temporal aspects of vision.
Amacrine cells are a diverse and functionally complex group of neurons located in the inner retina, playing a vital role in the processing and modulation of visual information. These cells are situated at the interface between bipolar cells and retinal ganglion cells (RGCs), and they interact extensively with both. Amacrine cells are known for their wide range of functions, diverse morphologies, and the numerous subtypes that contribute to their role in the visual system.
Unlike other retinal neurons, amacrine cells typically lack axons and communicate through their dendrites, which form extensive networks within the inner plexiform layer (IPL) of the retina. This unique structure allows them to integrate and modulate signals across a broad area of the retina, influencing the output of RGCs.
Amacrine cells are primarily involved in complex image processing tasks within the retina, including:
• Temporal and Spatial Integration: Amacrine cells integrate visual information over time and space, contributing to the detection of motion, changes in light intensity, and the overall dynamic aspects of vision. Their extensive dendritic networks enable them to process information across a wide area, enhancing the retina's ability to detect movement and temporal changes.
• Contrast Enhancement and Edge Detection: By modulating the input to RGCs, amacrine cells play a crucial role in enhancing contrast and facilitating edge detection. This is achieved through lateral inhibition, a process where amacrine cells inhibit neighboring bipolar cells and RGCs, sharpening the visual signal.
• Direction Selectivity and Motion Detection: Some subtypes of amacrine cells are specialized for detecting the direction of motion. These direction-selective amacrine cells are crucial for encoding the direction and speed of moving objects in the visual field.
• Light Adaptation and Sensitivity Regulation: Amacrine cells contribute to the retina's ability to adapt to different lighting conditions. They help regulate the sensitivity of bipolar cells and RGCs, ensuring that the retina can function effectively across a range of light intensities.
• Subtypes and Specialization: There are numerous subtypes of amacrine cells, each with unique morphologies and functions. Some subtypes are involved in specific aspects of color processing, while others modulate the activity of RGCs in response to specific patterns or types of movement. The diversity of amacrine cell subtypes allows for a high degree of specialization in retinal processing.
Transmission to the Brain
Retinal Ganglion Cells (RGCs)
The axons of RGCs form the optic nerve, which carries visual information from the eye to the brain. RGCs are responsible for the initial encoding and transmission of visual signals to the brain. Different types of RGCs process different aspects of the visual field, such as color, contrast, and movement.
Retinal Ganglion Cells (RGCs) are critical components in the visual processing pathway, situated at the final stage of the retinal circuitry. These cells play a pivotal role in converting visual information captured by photoreceptors into neural signals that can be interpreted by the brain. RGCs are not only conduits for transmitting visual data but also actively participate in the initial stages of visual processing. Their diversity in types and functions significantly contributes to the complexity and richness of visual perception.
The primary role of RGCs is to receive and integrate synaptic inputs from bipolar cells and amacrine cells within the retina. The integration of these inputs results in the generation of action potentials, or "spikes," which are then transmitted along the optic nerve to the brain. The nature of these action potentials encodes various aspects of the visual scene, such as light intensity, contrast, color, and motion.
One of the remarkable features of RGCs is their diversity. There are numerous subtypes of RGCs, each specialized for processing different aspects of the visual input. For example, some RGCs are particularly sensitive to specific wavelengths of light, contributing to color vision, while others are more responsive to changes in light intensity or motion. This specialization is essential for the parallel processing of various visual attributes, allowing the brain to construct a detailed and comprehensive visual representation.
RGCs are often classified based on their morphological characteristics, physiological responses, and the types of visual information they process. Commonly recognized types include:
• M-type (Magnocellular) RGCs: These cells are larger and have faster-conducting axons. They are highly sensitive to motion and temporal changes in the visual scene, making them crucial for detecting movement and providing input to the brain's motionprocessing pathways.
• P-type (Parvocellular) RGCs: These cells are smaller and have slower-conducting axons. They are highly sensitive to fine spatial details and color, playing a significant role in high-resolution vision and color discrimination.
• K-type (Koniocellular) RGCs: These cells, interspersed between the M and P layers in the LGN, are involved in processing specific color contrasts, such as blue-yellow.
In addition to these broad categories, there are other RGC subtypes specialized for various functions, such as detecting the overall luminance level, edge detection, and orientation. Some RGCs are specifically tuned to detect certain patterns of movement, such as radial or circular motion, contributing to the perception of complex dynamic scenes.
RGCs also exhibit a center-surround receptive field organization, a fundamental property that enhances the ability to detect contrasts and edges in the visual scene. The center-surround organization allows RGCs to respond preferentially to differences in light intensity between the central and peripheral regions of their receptive fields, which is critical for edge detection and spatial resolution.
Optic Nerve and Optic Chiasm
The optic nerves from both eyes meet at the optic chiasm, where fibers from the nasal (inner) halves of each retina cross to the opposite side of the brain. This arrangement allows visual information from both eyes to be integrated for binocular vision.
The optic nerve and optic chiasm are key structures in the visual pathway, playing essential roles in the transmission and processing of visual information from the eyes to the brain. While often perceived primarily as conduits for visual signals, these structures have unique features and involve specific neuron types that contribute to the overall process of visual perception.
The optic nerve, formed by the axons of retinal ganglion cells (RGCs), is the primary conduit through which visual information exits the eye and travels towards the brain. Each optic nerve corresponds to one eye, carrying the complete visual field perceived by that eye. The RGCs, which form the optic nerve, are diverse in type and function. They vary in their response to different aspects of the visual field, such as color, contrast, light intensity, and movement. This diversity allows the optic nerve to transmit a rich array of visual information for processing in the brain.
As the optic nerves approach the brain, they converge at a junction known as the optic chiasm. This structure is located at the base of the brain, just above the pituitary gland. The optic chiasm is a crucial point in the visual pathway, as it is where the partial decussation (crossing) of fibers from the two optic nerves occurs. Specifically, the axons from the nasal (inner) halves of each retina cross to the opposite side of the brain, while the axons from the temporal (outer) halves remain on the same side. This arrangement ensures that visual information from each eye's temporal field (peripheral vision) is processed on the same side of the brain, while information from the nasal field (central vision) is processed on the opposite side. This crossing is essential for binocular vision and depth perception, as it allows the brain to integrate and compare visual information from both eyes.
Although the optic nerve and optic chiasm are primarily considered as pathways for transmitting visual information, there is evidence suggesting some level of processing may occur within these structures. For instance, studies have indicated that certain types of RGCs can modulate their signal transmission based on ambient light conditions, which could be considered a form of preprocessing before the signals reach the brain. Additionally, the organization and crossing of fibers in the optic chiasm itself are vital for the correct mapping and integration of visual information in the brain.
Lateral Geniculate Nucleus (LGN) in the Thalamus
Neurons in the LGN receive input from RGCs and perform initial processing before relaying information to the primary visual cortex. The LGN has distinct layers that process different types of visual information, such as color and motion.
The Lateral Geniculate Nucleus (LGN), situated within the thalamus, plays a pivotal role in the visual processing pathway. As a primary relay center for visual information, the LGN serves as a critical inter mediary, transferring data from the retinal ganglion cells (RGCs) to the primary visual cortex (V1). The LGN's unique structure, intricate layer organization, and functional properties underscore its significance in the visual system.
Anatomically, the LGN is a bilaterally symmetrical structure, with each hemisphere of the brain housing an LGN that processes information from the contralateral visual field. This paired structure is distinguished by its six-layer composition in primates, including humans. Each layer receives inputs from either the contralateral or ipsilateral eye, with layers 1, 4, and 6 associated with the former and layers 2, 3, and 5 with the latter.
The layers of the LGN are further categorized based on the type of RGC input they receive. The magno-cellular layers (layers 1 and 2) are connected to larger, M-type ganglion cells and are integral to processing motion and coarse spatial information. Conversely, the parvocellular layers (layers 3 through 6) receive inputs from smaller, P-type ganglion cells and are responsible for processing fine spatial details and color. Some primates also possess koniocellular layers, which are interspersed between the main layers and receive inputs from K-type ganglion cells. These layers are believed to be involved in processing blue-yellow color contrast and other aspects of visual information.
Functionally, the LGN is not merely a passive relay station but is actively involved in several aspects of visual processing. It enhances contrast, integrates spatial and temporal visual signals, and plays a role in visual attention regulation and sensory information filtering. The LGN's ability to modulate the flow of visual data to the cortex based on attentional demands and arousal state is particularly notable. Additionally, it maintains a retinotopic map, preserving the spatial organization of the retina in its projection, which is crucial for spatial coherence in visual perception.
The neural circuitry within the LGN is characterized by receptive fields influenced by the center-surround organization of RGCs. This arrangement allows the LGN to effectively detect luminance contrast and facilitate edge detection in visual scenes. Moreover, the LGN receives not only feedforward input from RGCs but also feedback connections from the visual cortex and other brain regions. This suggests its involvement in higher-level visual processing and the cognitive influences on perception.
In its role within the visual pathways, the LGN is instrumental in segregating and processing different visual information aspects, such as motion, color, and form. After processing in the LGN, visual information is conveyed to the primary visual cortex via the optic radiations, establishing the LGN as a critical gateway for cortical visual processing.
Primary Visual Cortex (V1)
V1
• Neurons in the primary visual cortex, located in the occipital lobe of the brain, are responsible for further processing and interpreting visual information. Cells in V1, such as simple cells, complex cells, and hypercomplex cells, extract features like edges, orientation, and movement from the visual scene.
• V1 is organized retinotopically, meaning there is a mapping of the visual field onto the cortical surface.
The primary visual cortex (V1), also known as the striate cortex or area 17, is a critical region in the brain for the processing of visual information. Located in the occipital lobe, V1 is the first cortical area to receive visual input from the eyes via the lateral geniculate nucleus (LGN) of the thalamus. This area is fundamental in the hierarchical processing of visual stimuli, and it exhibits unique features and specialized neuron types that are essential for the interpretation and perception of visual information.
Structural Organization
• V1 is characterized by its distinct layered structure, typically comprising six layers, each with specific types of neurons and functions. These layers receive and process visual information, with different layers having different connections and roles in visual processing.
• The surface of V1 exhibits a striped appearance due to the presence of stria of Gennari, a band of myelinated axons, which is a distinguishing feature of this region.
Functional Specialization
• Neurons in V1 are specialized for processing various aspects of the visual scene, such as orientation, spatial frequency, color, and motion. This specialization is evident in the properties of different types of neurons found in V1.
• V1 maintains a retinotopic map, meaning that there is a systematic spatial correspondence between the retina and the representation of the visual field in the cortex. This mapping is essential for preserving the spatial structure of the visual scene.
Neuron Types in V1
• Simple Cells: These neurons respond best to bars or edges of specific orientations within their receptive fields. They have distinct excitatory and inhibitory regions and are thought to be critical for edge detection and orientation selectivity.
• Complex Cells: Complex cells also respond to specific orientations but have larger receptive fields and are less sensitive to the exact position of the stimulus within the field. They are important for detecting moving edges and patterns.
• Hypercomplex Cells: Also known as end-stopped cells, hypercomplex cells are sensitive to the length of a stimulus in addition to its orientation. They play a role in perceiving the ends of objects and detecting corners and angles.
Columnar Organization
V1 exhibits a columnar organization, where neurons with similar properties (such as orientation preference) are arranged in vertical columns that extend through the layers of the cortex. This organization facilitates the integration of information about specific visual attributes.
Unusual Features and Processing
• V1 is involved in the initial stages of parallel processing of visual information, where different features of the visual scene are processed simultaneously in separate pathways. For instance, the dorsal stream (processing "where" and "how") and the ventral stream (processing "what") originate from
V1. • The processing in V1 is influenced not only by feedforward input from the LGN but also by feedback from higher-order visual areas and other cortical regions. This feedback can modulate the activity of V1 neurons, influencing perception and attention.
V1 Hands off to Vn: The processed visual information from V1 is sent to secondary and tertiary visual areas for higher-level processing. These areas include V2, V3, V4, and V5/MT (middle temporal), each specialized for processing different aspects of vision such as color (V4) and motion (V5/MT).
V2
The V2, or Secondary Visual Cortex, is a crucial area in the cerebral cortex for the processing of visual information. Located adjacent to the primary visual cortex (V1) in the occipital lobe, V2 serves as a key relay point in the visual processing pathway, receiving and further refining the visual inputs from V1. This area exhibits unique features and specialized neuron types that enable it to contribute significantly to various aspects of visual perception.
Structural and Functional Organization
• V2 is characterized by its distinct cytoarchitectonic structure and is larger compared to V1. It maintains a retinotopic organization, similar to V1, ensuring the spatial mapping of the visual field.
• The area is divided into different functional zones, often referred to as stripes: thin stripes, thick stripes, and inter-stripes. Each of these stripes is specialized for processing different types of visual information, contributing to the modular organization of V2.
Processing in V2
• V2 plays a pivotal role in further processing the visual information relayed from V1. This includes the refinement of basic visual attributes such as orientation, spatial frequency, and color, as well as the integration of these attributes to perceive more complex patterns and forms.
• V2 is involved in processing both low-level and higher-order visual features, including the interpre tation of illusory contours, figure-ground segregation, and depth perception through binocular disparity.
Neuron Types and Functional Properties
• Neurons in V2 exhibit diverse response properties and are more complex compared to those in V1. These neurons can be sensitive to specific orientations, spatial frequencies, and colors, and they also respond to more complex stimuli such as angles, corners, and curved lines.
• V2 contains neurons that are specialized in processing binocular disparity, which is crucial for stereoscopic depth perception. These neurons help in constructing a three-dimensional representation of the visual environment.
Role in Visual Pathways
• V2 serves as an important intermediary in both the dorsal and ventral streams of visual processing. The dorsal stream, projecting towards the parietal cortex, is involved in processing spatial location and motion, while the ventral stream, projecting towards the temporal cortex, is involved in object recognition and form analysis.
• The integration of information in V2 contributes to the segregation of these two streams, each of which processes different aspects of the visual scene.
Unusual Features and Connectivity
• V2 exhibits complex intrinsic connectivity, with extensive horizontal connections within the cortex that facilitate the integration of information across different functional zones.
• V2 also receives feedback inputs from higher-order visual areas, indicating its involvement in top-down processing and the influence of cognitive factors such as attention and expectation on visual perception.
V3
The V3, or Tertiary Visual Cortex, is an important region in the brain's visual processing network. Located in the occipital lobe and adjacent to the primary (V1) and secondary (V2) visual cortices, V3 plays a significant role in the interpretation and integration of visual information. This area is distinguished by its unique neuron types, functional properties, and contributions to specific aspects of visual perception.
Structural and Functional Organization
• V3 is part of the larger visual processing system and maintains a retinotopic organization, similar to V1 and V2, which helps in mapping the visual field spatially onto the cortical surface.
• The functional role of V3 is diverse, and it is believed to contribute to both the dorsal and ventral streams of visual processing. The dorsal stream, associated with spatial awareness and motion processing, and the ventral stream, associated with object recognition and form processing, both receive inputs from V3.
Processing in V3
• V3 is involved in the processing of complex visual stimuli, including the perception of motion, depth, and form. It plays a role in integrating visual information from V1 and V2 and further elaborating on this information.
• V3 neurons contribute to the understanding of global motion patterns and the perception of the speed and direction of moving objects. This is crucial for navigating dynamic environments and interacting with moving objects.
Neuron Types and Functional Properties
• Neurons in V3 exhibit diverse response properties, with some neurons being particularly sensitive to specific orientations, spatial frequencies, and motion directions. These neurons help in detecting and inter preting complex visual patterns and motion.
• V3 contains neurons that are involved in the processing of binocular disparity, contributing to stereoscopic depth perception. This feature allows for the perception of three-dimensional depth and structure in the visual scene.
Role in Visual Pathways
• V3 serves as an important node in the visual processing pathways, linking the initial processing in V1 and V2 with more complex interpretation in higher-order visual areas.
• The integration of information in V3 is critical for the segregation and specialization of the dorsal and ventral streams, each processing different aspects of the visual scene.
Unusual Features and Connectivity
• V3 exhibits complex intrinsic connectivity, with extensive horizontal connections within the cortex. These connections facilitate the integration of information across different areas of V3, contributing to the holistic processing of visual stimuli.
• V3 also receives feedback inputs from higher-order visual areas, indicating its involvement in top-down processing. This suggests that cognitive factors such as attention, expectation, and prior experience can influence visual perception at the level of V3.
V4
V4 is a significant area within the visual cortex, playing a crucial role in the processing of visual information, particularly in the aspects of color and form perception. Situated along the ventral stream of the visual pathway, which is involved in object recognition and detailed visual analysis, V4 exhibits unique neuron types and functional properties that contribute to its specialized role in visual perception.
Functional Specialization
• V4 is primarily involved in processing color and form, including complex geometric patterns and object features. This specialization is essential for the perception of a detailed and colorful visual world.
• The area is also implicated in the processing of spatial attention and the integration of visual information with other sensory modalities, contributing to the creation of a coherent sensory experience.
Neuron Types and Properties
• Neurons in V4 exhibit diverse response characteristics, with many being highly sensitive to specific aspects of visual stimuli. This includes sensitivity to color, shape, orientation, and spatial frequency.
• Color processing in V4 is particularly notable. Neurons here are involved in color constancy, the ability to perceive consistent colors under varying lighting conditions. These neurons can integrate information about the spectral properties of light and the context of the visual scene to maintain stable color perception.
• Additionally, V4 neurons contribute to the perception of complex shapes and patterns, responding to a range of geometric forms and visual textures.
Role in Visual Processing Pathways
• V4 serves as an intermediate processing stage in the ventral stream, receiving inputs from lower-level visual areas like V2 and V3 and sending outputs to higher-order areas involved in object recognition and memory, such as the inferior temporal cortex.
• The integration of visual information in V4 is critical for the recognition and interpretation of objects and scenes, bridging the gap between basic visual features and complex perceptual constructs.
Unusual Features and Connectivity
• V4 exhibits a high degree of intra-areal connectivity, with extensive horizontal connections that facilitate the integration of information across different parts of the visual field. This connectivity is important for the holistic perception of objects and scenes.
• V4 also receives feedback inputs from higher-order visual and cognitive areas, suggesting that perception in V4 is influenced by factors such as attention, expectation, and experience.
• V4 offers valuable insights into the neural mechanisms underlying color perception, object recognition, and the integration of visual information, and it has important implications for understanding visual perception, cognition, and disorders affecting vision.
V5/MT
V5/MT, also known as the Middle Temporal visual area, is a crucial region in the visual cortex that specializes in the processing of motion. Located along the dorsal stream of the visual processing pathway, V5/MT plays a pivotal role in perceiving and interpreting motion, an essential aspect of visual cognition and navigation. The unique neuron types and functional properties of V5/MT underscore its significance in the neural network responsible for visual perception.
Functional Specialization
• V5/MT is primarily dedicated to the detection and analysis of motion within the visual field. It processes information about the direction, speed, and coherence of moving objects, contributing to the perception of fluid motion and dynamic scenes.
• The area is also involved in the perception of motion in depth, which is crucial for navigating and interacting with a threedimensional environment.
Neuron Types and Properties
• Neurons in V5/MT exhibit specialized response characteristics, with many being selectively sensitive to specific directions and speeds of motion. This selectivity allows V5/MT to encode detailed information about the dynamics of moving objects.
• V5/MT contains direction-selective neurons, which respond preferentially to movement in particular directions. This feature is critical for discerning the trajectory and orientation of moving objects.
• Neurons in V5/MT also contribute to the processing of motion coherence, the ability to detect uniform motion patterns within a field of random motion, which is important for distinguishing moving objects from their backgrounds.
Role in Visual Processing Pathways
• V5/MT is a key component of the dorsal stream, which is associated with the processing of spatial location and movement. It receives inputs from earlier visual areas, including V1 and V2, and integrates this information to construct a comprehensive representation of motion.
• The output from V5/MT is sent to higher-order areas involved in motion analysis and visually guided actions, such as the posterior parietal cortex.
Unusual Features and Connectivity
• V5/MT exhibits a high degree of intra-areal connectivity, with extensive horizontal connections that enable the integration of motion information across different regions of the visual field. This connectivity is essential for the holistic perception of motion and the coordination of visually guided actions.
• V5/MT is also characterized by its strong interconnections with other areas of the dorsal stream, as well as feedback connections from higher-order cognitive and motor regions. These connections suggest that the processing of motion in V5/MT is influenced by a range of factors, including attention, prediction, and behavioral context.
Involvement in Visual Illusions and Disorders
• V5/MT is noteworthy for its involvement in certain visual illusions related to motion, such as the perception of movement in static images. These illusions highlight the complex neural mechanisms underlying motion perception.
• Additionally, abnormalities in V5/MT have been implicated in motion perception disorders, providing insights into conditions like akinetopsia, where individuals have difficulty perceiving motion.
• V5/MT offers valuable insights into the neural mechanisms underlying motion perception and has important implications for understanding visual perception, cognition, and disorders affecting motion processing in the visual system.
These higher visual areas are interconnected and also communicate with other parts of the brain, such as the parietal and temporal lobes, for integrating visual information with memory, spatial awareness, and other sensory modalities.
Visual processing involves a highly integrated and complex network of neurons and brain regions, each contributing to the creation of a coherent and detailed visual perception. This process exemplifies the remarkable capabilities of the nervous system in processing and interpreting sensory information.
Structure of Neurons
A "typical" neuron, the fundamental building block of the nervous system, is characterized by four distinct regions, each playing a crucial role in the neuron's function. Understanding the structure and function of these regions is key to comprehending how neurons communicate and process information.
The first region is the cell body, also known as the soma. The cell body is the metabolic and organizational hub of the neuron, responsible for maintaining the cell's health and functionality. It houses the nucleus, which contains the cell's genetic material and controls protein synthesis. The cell body also functions as the neuron's manufacturing and recycling plant, where essential neuronal proteins and other components are synthesized and processed. This includes the production of neurotransmitters, enzymes, and structural proteins necessary for the neuron's operation.
The second and third regions of a neuron are its processes, which are structures that extend away from the cell body. These processes serve as conduits for signals to flow to or away from the cell body. The incoming signals from other neurons are typically received through the neuron's dendrites. Dendrites are branched, tree-like structures that provide a large surface area for receiving synaptic inputs from other neurons. Each neuron may have numerous dendrites, each covered in synaptic sites where neurotransmitters from other neurons can bind and influence the neuron's activity. The dendrites play a critical role in integrating these incoming signals and transmitting them to the cell body.
The outgoing signal to other neurons flows along the neuron's axon. A neuron typically has only one axon, but this axon can branch extensively to communicate with multiple target neurons. The axon is a long, slender projection that conducts electrical impulses, known as action potentials, away from the cell body. The axon's length and myelination (in some neurons) enable rapid transmission of these electrical signals over long distances.
The fourth distinct region of a neuron is found at the end of the axon: the axon terminals, also known as terminal boutons. These structures contain synaptic vesicles filled with neurotransmitters, the chemical messengers used for communication between neurons. When an action potential reaches the axon terminals, it triggers the release of neurotransmitters into the synaptic cleft, the small gap between neurons. The neurotransmitters then bind to receptor sites on the dendrites or cell bodies of the receiving neuron, facilitating the transmission of signals across the synapse.
Neurotransmitters play a crucial role in neuronal communication, allowing signals to flow from one neuron to the next at chemical synapses. The type and amount of neurotransmitter released, along with the specific receptors on the receiving neuron, determine the nature of the signal transmitted — whether it is excitatory or inhibitory, for instance. This chemical communication is fundamental to the functioning of neural networks and underlies all neural processes, from basic reflexes to complex cognitive tasks.
Types of Neurons This diversity is reflected in their morphology, electrophysiological properties, and the roles they play within neural circuits. The different types of neurons have distinct characteristics that affect their individual behavior and contributions to overall neural function. Here, we explore some major types of neurons and how their unique features influence their behavior.
Sensory Neurons
• Sensory neurons, also known as afferent neurons, are responsible for converting external stimuli from the environment into internal electrical impulses. These neurons are typically found in sensory organs such as the eyes, ears, skin, and nose.
• The unique feature of sensory neurons is their ability to respond to specific types of sensory inputs, such as light, sound, touch, or chemical signals. Their behavior is characterized by the translation of these stimuli into nerve signals that are relayed to the brain or spinal cord for processing.
Motor Neurons
• Motor neurons, or efferent neurons, carry signals from the central nervous system to muscles and glands, facilitating movement and action. They are key players in the motor system and are involved in both voluntary and involuntary actions.
• The behavior of motor neurons is marked by their ability to stimulate muscle contraction or gland secretion. They receive signals from the brain or spinal cord and translate them into specific actions, such as muscle movement or glandular responses.
Interneurons
• Interneurons are neurons that are located entirely within the central nervous system and serve as connectors between other neurons. They are the most numerous type of neuron and play critical roles in information processing and signal integration.
• The behavior of interneurons is diverse, as they are involved in various functions such as reflexes, neural oscillations, and complex cognitive processes. Their ability to form extensive networks allows them to integrate signals from multiple sources, modulate neural circuits, and influence the output of motor neurons.
Pyramidal Neurons
Pyramidal neurons are a type of excitatory neuron found in the cerebral cortex and hippocampus. They are characterized by a pyramid-shaped cell body and a single long axon. The behavior of pyramidal neurons is crucial for cognitive functions such as learning, memory, and decision-making. They are involved in the transmission of signals within the cortex and between the cortex and other brain regions. The unique structure of pyramidal neurons, with their extensive dendritic trees, allows them to receive and integrate numerous inputs from other neurons. The double pyramidal cell, a specific type of neuron found in the cerebral cortex, exhibits unique structural and functional characteristics. While there isn't direct information available on "double pyramidal cells" specifically, insights can be drawn from the general understanding of pyramidal cells in the cerebral cortex.
Structure and Characteristics of Pyramidal Cells Morphology
• Pyramidal cells are the most abundant type of neuron in the cerebral cortex.
• They are characterized by a pyramid-shaped cell body, a large apical dendrite extending towards the cortical surface, multiple basal dendrites, and an axon that projects to various cortical and subcortical areas.
Dendritic Structure
The dendrites are covered in spines, which are sites of synaptic input.
• The apical dendrite typically branches as it ascends and may have additional oblique branches.
• The complexity of dendritic arborization varies among pyramidal cells in different cortical areas and among species, reflecting their functional diversity
Axonal Projections
• Axons can extend over long distances, facilitating communication between different brain regions.
• Pyramidal cells in different cortical layers have distinct projection patterns. For example, cells in layer V often project to subcortical structures, while those in layer III might project to other cortical areas.
Electrophysiological Properties
• Pyramidal cells exhibit diverse firing patterns, including regular spiking and bursting, which are crucial for information processing and transmission in neural circuits.
• They typically exhibit excitatory neurotransmission, primarily using glutamate as a neurotransmitter.
Functional Role
• Pyramidal neurons play a key role in cognitive functions like sensory perception, motor control, and complex thought processes.
• They are involved in synaptic plasticity mechanisms, which are fundamental for learning and memory.
Variations Among Species
Comparative studies show that pyramidal cells vary markedly in structure among different species and cortical regions, suggesting adaptations to specific cognitive functions.
Implications of Structural and Functional Diversity
The diversity in the structure and function of pyramidal cells, including those with atypical features like double pyramidal cells, underscores the complexity of neural processing in the cerebral cortex. This diversity allows for a range of computational capabilities and adaptive responses to environmental inputs. Understanding these variations can provide insights into the cellular basis of cognitive functions and the pathophysiology of neurological disorders.
The available literature provides a detailed view of the pyramidal neurons’ characteristics, although specific information on "double pyramidal cells" as a distinct subtype may require further research for precise details.
Certainly, let's delve deeper into the unusual characteristics and firing patterns of double pyramidal cells:
Unusual Characteristics
Dendritic Orientation: One of the most unusual characteristics of double pyramidal cells is their dendritic structure, with two opposing dendritic tufts. This contrasts with typical pyramidal neurons that have a single apical dendrite extending towards the cortical surface. The bipolar dendritic orientation of double pyramidal cells allows them to potentially integrate information from different cortical layers or areas.
Axonal Projections: The axons of double pyramidal cells may have distinct projection patterns compared to other pyramidal neurons. They might innervate local as well as distant cortical regions, contributing to both local circuitry and long-range cortical connections.
Synaptic Specificity: These cells might show specific patterns of synaptic connectivity, receiving inputs from distinct types of neurons or specific cortical layers, which could influence how they process and relay information.
Molecular and Genetic Markers: There may be unique molecular or genetic markers that distinguish double pyramidal cells from other neuron types, which can be important for understanding their development, function, and involvement in diseases.
Firing Patterns
Response to Stimulation: The firing patterns of double pyramidal cells in response to synaptic stimulation can provide insights into their functional role. These cells might exhibit distinct responses to excitatory and inhibitory inputs compared to other pyramidal neurons.
Spontaneous Activity: The spontaneous firing rate and pattern of these neurons, observed in the absence of external stimuli, can indicate their baseline activity and role in maintaining cortical network dynamics.
Adaptation and Plasticity: The ability of double pyramidal cells to adapt their firing patterns in response to changes in synaptic input or environmental conditions is crucial for understanding their role in learning and memory processes.
Pathological Conditions: Alterations in the firing patterns of double pyramidal cells can be associated with various neurological conditions. For instance, changes in their excitability or responsiveness could be linked to epilepsy, autism, or other cortical dysfunctions.
Comparative Analysis: Comparing the firing patterns of double pyramidal cells with those of other cortical neurons can reveal how these unique cells contribute to the overall function of the cerebral cortex.
Studying the unusual characteristics and firing patterns of double pyramidal cells is key to understanding their contribution to cortical processing and functionality. These aspects are particularly relevant in the context of sensory integration, cognitive processing, and the potential involvement of these cells in neurological disorders. As research progresses, it is likely that more detailed insights into the unique properties of double pyramidal cells will emerge, enhancing our understanding of cortical neurobiology.
Purkinje Neurons
• Purkinje neurons are found in the cerebellum and are among the largest neurons in the brain. They have an elaborate dendritic arbor that forms a flat, fan-like structure.
• The behavior of Purkinje neurons is central to motor coordination and balance. They receive inputs from various sources, including sensory and motor systems, and play a key role in refining motor movements. The distinctive architecture of their dendritic trees enables them to process a vast amount of information and modulate the activity of the cerebellar circuits.
Inhibitory Neurons
• Inhibitory neurons, such as basket cells and chandelier cells, primarily use neurotransmitters like gamma-aminobutyric acid (GABA) to inhibit the activity of other neurons. They are essential for regulating the excitability of neural circuits.
• The behavior of inhibitory neurons is characterized by their ability to control and balance the excitation within neural networks. They prevent excessive neuronal firing and maintain the stability of neural circuits, which is crucial for preventing disorders like epilepsy.
Sensory neurons specialize in translating external stimuli into neural signals, while motor neurons are involved in eliciting responses and actions. Interneurons, with their extensive connectivity, integrate and modulate signals within neural circuits. Pyramidal neurons, primarily found in the cerebral cortex, play key roles in higher cognitive functions. Purkinje neurons, with their elaborate dendritic trees, are central to motor coordination in the cerebellum. Inhibitory neurons, such as basket and chandelier cells, regulate neural excitability and maintain the balance of excitation and inhibition in the brain.
Each type of neuron contributes uniquely to the overall functioning of the nervous system, and their individual behaviors are shaped by their specific structural and functional attributes. Understanding the diverse types of neurons and their distinct roles is essential for unraveling the complexities of neural circuits and for advancing our knowledge of brain function and neural disorders. The intricate interplay among different neuron types facilitates the remarkable capabilities of the brain, from basic sensory processing to complex cognitive tasks.
Neural Response
In response to a voltage input, a biological neuron goes through a series of processes that include spike production (action potential generation), decay of sensitivity with repeated firings (accommodation or adaptation), and changes in the threshold. Here's a detailed description of each step:
Response to Voltage Input When a voltage input, such as synaptic input or an externally applied voltage changes, depolarizes a neuron's membrane potential, it moves closer to the firing threshold.
Spike Production (Action Potential Generation) If the depolarization is strong enough to reach the neuron's firing threshold, voltage-gated sodium (Na (^+)) channels open, causing a rapid influx of Na (^+) ions. This influx further depolarizes the membrane, leading to the rapid rising phase of the action potential. After reaching a peak, the membrane begins to repolarize as voltage-gated potassium (K (^+)) channels open, allowing K (^+) ions to exit the neuron. This repolarization returns the membrane potential towards the resting level. The neuron may briefly hyperpolarize (undershoot the resting potential) before returning to its resting state, at which point it's ready to respond to new inputs.
Decay of Sensitivity with Repeated Firings (Adaptation) With repeated or sustained stimulation, a neuron can exhibit a decrease in responsiveness, known as accommodation or adaptation. This is often due to the activation of various ion channels that counteract depolarization or due to inactivation of Na (^+) channels. This process makes it harder for the neuron to reach the firing threshold with subsequent stimuli, effectively increasing the threshold or reducing the neuron's excitability temporarily.
Threshold Changes The firing threshold of a neuron is not static and can change based on the neuron's recent activity and the balance of excitatory and inhibitory inputs it receives. After repetitive firing, certain ion channels (like calcium-activated potassium channels) may be activated, which can hyperpolarize the membrane or stabilize it, making it harder to depolarize to the threshold. Conversely, certain neuromodulators or synaptic inputs can lower the firing threshold, making the neuron more likely to fire in response to subsequent inputs.
Global Mechanisms
Global mechanisms for neural control are crucial in regulating and coordinating neural activity across various regions of the brain and nervous system. These overarching processes and systems play an essential role in maintaining the balance and functionality of neural circuits, ensuring that the nervous system operates in a coordinated and adaptive manner. Several key global mechanisms work in concert to achieve this regulation:
Neuromodulation is a significant global mechanism that involves the release of neuromodulators, chemicals that can modify the activity of neurons and neural circuits across the brain. Neuromodulators, such as dopamine, serotonin, acetylcholine, and norepinephrine, differ from neurotransmitters in that they often have widespread effects, impacting multiple brain regions and neural pathways simultaneously. This allows neuromodulators to play a crucial role in modulating a range of brain functions, including mood, arousal, attention, and motivation.
Hormonal regulation, mediated by the endocrine system, is another global mechanism that exerts a broad influence on brain function and behavior. Hormones like cortisol, estrogen, testosterone, and thyroid hormones can affect a wide array of neural processes, from brain development and neural plasticity to mood regulation and stress responses. These hormones are released into the bloodstream and can affect neurons and neural circuits throughout the brain and body, providing a systemic means of neural regulation.
The reticular activating system (RAS), located in the brainstem, is essential in regulating wakefulness and alertness. The RAS sends projections to various parts of the brain, including the thalamus and cortex, influencing the overall level of consciousness and attention. It acts as a gatekeeper for sensory information, filtering incoming stimuli, and prioritizing those that require attention. By maintaining an optimal level of arousal and responsiveness, the RAS ensures that individuals remain attentive and responsive to their environment.
The autonomic nervous system (ANS), comprising the sympathetic and parasympathetic divisions, is a key player in global neural control. The ANS regulates involuntary bodily functions such as heart rate, blood pressure, digestion, and respiratory rate. It operates globally to maintain homeostasis and to respond appropriately to stressors, modulating the activity of various organs and systems in response to internal and external stimuli.
Circadian rhythms, the 24-hour cycles that regulate physiological and behavioral processes, including sleep-wake cycles, hormone release and metabolism, also constitute a global control mechanism. The suprachiasmatic nucleus (SCN) in the hypothalamus acts as the body's master circadian clock, coordinating these rhythms across different tissues and organs. This regulation ensures that internal processes are synchronized with external environmental cues, such as light and darkness.
Additionally, higher brain regions, particularly the prefrontal cortex, exert top-down control over other parts of the brain. This control allows for the regulation of complex cognitive processes, emotional responses, and goal-directed behaviors. Through topdown mechanisms, the brain can prioritize and modulate sensory information, influence decision-making processes, and regulate emotional and behavioral responses based on the context and individual goals.
Global mechanisms for neural control encompass a range of processes and systems that collectively ensure the coordinated and adaptive functioning of the nervous system. These mechanisms include neuromodulation, hormonal regulation, the reticular activating system, the autonomic nervous system, circadian rhythms, and top-down control from higher brain regions. Together, they maintain homeostasis, regulate physiological and psychological processes, and enable complex behaviors and cognitive functions. The global systems highlight the remarkable ability of the nervous system to integrate and respond to a vast array of stimuli.
Prior Work in Video Processing with Spike Neurons
The use of Liquid State Machines (LSMs) in video processing has been explored in various studies, focusing on different aspects such as movement prediction, real-time processing, and action recognition. Here are some key papers on this topic:
• Movement Prediction from Real-World Images: Introduced an approach using LSM combined with supervised learning algorithms to predict object trajectories, specifically ball trajectories, in robotics based on video data from a camera mounted on a robot.
• Real-Time Processing and Spatio-Temporal Data: Discussed a digital neuromorphic design of LSM for real-time processing of spatiotemporal data, focusing on its potential in tasks dependent on a system's behavior over time. This work highlights the capability of LSMs in handling complex data streams, such as those found in video processing.
• Parallelized LSM for Action Detection in Videos: Presented a novel Parallelized LSM (PLSM) architecture for classifying unintentional actions in video clips. This study demon started the effectiveness of PLSM in outperforming both self-supervised models and traditional deep learning models in video processing tasks.
• Deep Liquid State Machine (D-LSM) for Computer Vision: Proposed the Deep Liquid State Machine (D-LSM), which integrates the capabilities of recurrent spiking networks with deep architectures. This model aims to enhance the extraction of spatio-temporal features from input, relevant to video processing applications.
These studies showcase the diverse applications and advancements of LSMs in the field of video processing, highlighting their potential in predicting movements, processing real-time data, and detecting actions in video streams.
Spike Modulated Artificial Neuron
Membrane Potential
The neuron generates spikes in response to incoming voltage. This voltage is considered, in biological terms, the electrical potential difference across the neuronal membrane, which results from the distribution of ions (such as sodium, potassium, chloride, and calcium) between the inside and outside of the neuron. From our biomimicry perspective, this is a continuous value.
The membrane potential
V (t) = max(0, V (t − 1) + I(t) − δ ⋅ V (t − 1))
Where:
•V (t) is the membrane potential at time t.
• V (t − 1) is the membrane potential from the previous time step.
• I is the voltage input.
• δ is the membrane decay rate.
Spiking versus Graded Potentials
In the visual pathway, different types of cells use distinct mechanisms for signaling, with some utilizing action potentials or "spikes," and others relying on graded potentials. Here's an overview of how various cells in the visual pathway use these signaling mechanisms:
Cells Using Action Potentials (Spikes)
• Retinal Ganglion Cells (RGCs): RGCs use action potentials to transmit visual information from the retina to the brain. The spikes generated by RGCs carry the processed visual information along the optic nerve to the lateral geniculate nucleus (LGN) and then to the visual cortex.
• Neurons in the Lateral Geniculate Nucleus (LGN): Neurons in the LGN use action potentials to relay visual information from the optic nerve to the visual cortex.
• Neurons in the Visual Cortex (V1, V2, V3, V4, V5/MT, etc.): Neurons in the various areas of the visual cortex, including the primary visual cortex (V1) and higher visual areas, use action potentials to process and transmit visual information within the cortex and to other brain regions.
Cells Using Graded Potentials
• Photoreceptors (Rods and Cones): Photoreceptors use graded potentials to respond to light. When activated by light, they undergo hyperpolarization, which modulates the release of neurotransmitters to bipolar cells. Photoreceptors do not generate action potentials.
• Bipolar Cells: Bipolar cells in the retina also use graded potentials to transmit signals from photoreceptors to retinal ganglion cells. The amount of neurotransmitter release by bipolar cells is proportional to the degree of their membrane potential change.
• Horizontal Cells: Horizontal cells use graded potentials to modulate the input from photoreceptors to bipolar cells, contributing to lateral inhibition and contrast enhancement in the retina.
• Amacrine Cells: Amacrine cells use graded potentials to influence the activity of bipolar cells and retinal ganglion cells. They play a role in various aspects of visual processing, such as motion detection, adaptation, and temporal integration.
Graded potentials, unlike action potentials, do not follow the all-ornothing principle and vary in magnitude. The cells that use graded potentials typically modulate the release of neurotransmitters based on the degree of their membrane potential changes, allowing for more subtle and continuous modulation of signals.
Retinal ganglion cells and neurons within the lateral geniculate nucleus and visual cortex predominantly use action potentials (spikes) for transmitting visual information. In contrast, photoreceptors, bipolar cells, horizontal cells, and amacrine cells in the retina rely on graded potentials for their signaling mechanisms. This distinction in signaling methods plays a crucial role in the processing and transmission of visual information from the retina to the higher visual centers in the brain.
Spiking Neurons
The operation of spike neurons, or neurons that use action potentials for communication, can be described using various mathematical models. One of most well-known and widely used models is the leaky-integrate-and-fire (LIF) model. The LIF model provides a simplified way to represent the membrane potential dynamics of a neuron and its firing of action potentials (spikes). Here's the basic mathematical notation for the LIF model:
Let V(t) be the membrane potential of the neuron at time t. The dynamics of V(t) in the LIF model are given by the following differential equation:
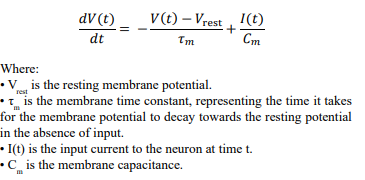
The neuron fires an action potential (spike) whenever reaches a certain threshold value Vthresh. After firing, the membrane potential is reset to a reset potential Vreset, and the neuron enters a refractory period during which it cannot fire another spike.
The LIF model is a simplification of real neuronal dynamics, but it captures the essential features of how neurons integrate incoming signals and generate spikes. More complex models, such as the Hodgkin-Huxley model or the FitzHugh-Nagumo model, provide more detailed and biophysically accurate descriptions of neuronal spiking behavior. However, the LIF model remains popular due to its simplicity and ability to capture the key aspects of neuronal spiking in a computationally efficient manner.
Hodgkin-Huxley Model
The Hodgkin-Huxley model is a mathematical model that describes how action potentials in neurons are initiated and propagated. It was developed by Alan Lloyd Hodgkin and Andrew Huxley in 1952, based on their experiments with the giant axon of the squid.
The model is widely used in neuroscience and is considered a fundamental contribution to understanding neuronal function.
The Hodgkin-Huxley model is based on a set of differential equations that describe the flow of ionic currents through the membrane of a neuron. The key components of the model include the membrane potential, ion channels, and the dynamics of sodium and potassium ions. The model represents the neuron's membrane as an electrical circuit with capacitors and voltage-gated ion channels.
Here are the primary equations: Membrane equation:
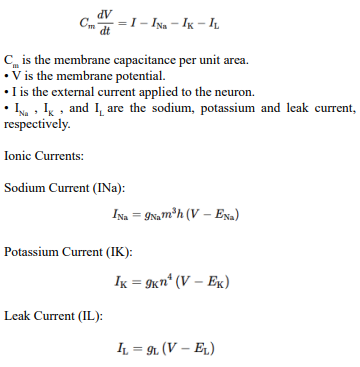
Where:
• gNa, gK , and gL are the maximum conductance for sodium, potassium, and leak channels, respectively. • ENa, EK, and EL are the reversal potentials for sodium, potassium, and leak channels, respectively. • m, h, n are gating variables that represent the probability of channel opening
The gating variables m , h , or n and αx(V ) and are voltagedependent rate constants for channel opening and closing of the voltage-gated ion channels. For each gating variable, the rate constants αx(V ) and βx(V ) are functions of the membrane potential V . They are not constants in the traditional sense but are rather functions that change with the voltage across the neuron's membrane. The functions are typically described by exponential or sigmoidal equations that were fitted to the experimental data.
The general form of the rate equations is
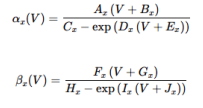
Where: Ax ,Bx ,Cx ,Dx ,Ex ,Fx ,Gx ,Hx ,Ix , and Jx are coefficients that were empirically determined for each gating variable. The specific form of these equations and their coefficients vary for the different gating variables (m, h, and n)
For example, the rate constants for the m gating variable, which is involved in the activation of the sodium channel, might take a form like this:
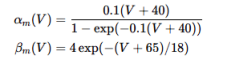
The forms of αh (V ), βh (V ), αn (V ), and βn (V ) would be different but would follow a similar structure, with coefficients fitted to the experimental data specific to each gating variable
We can condense the membrane and ionic equations as:
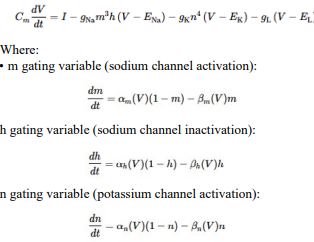
Each gating variable (m, h, n) has its own kinetics, governed by voltage-dependent rate constants and βx (V). These variables represent the probability of ion channel states (open, closed, inactivated) and are crucial for determining the ionic currents across the neuron's membrane.
This can be approximated by Euler's method, but the RungeKutta method is more precise. It may be expressed by calculating four estimates for the slope at each time step and take a weighted average to update the variables. This changes the membrane
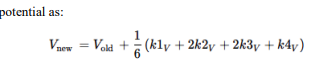
Where:
• Vold is the current value of the membrane potentia
• k1V, k2V, k3V, and k4V are the four estimates of the slope of V at different points within the time step interval, calculated using the right-hand side of the membrane equation
• 1/6 (k1V + 2k2V + 2k3V + k4V) is the weighted average of these slopes, which gives the approximate change in V over the time step
Update the membrane potential V by calculating the four estimates for V as:
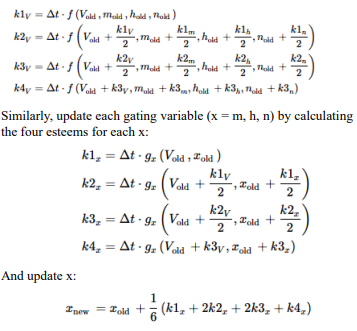
The RK4 method involves evaluating the derivative (or slope) of the function at the beginning of the interval (k1)), at the midpoint (k2 and k3), and at the end of the interval (k4), and then taking a weighted average of these slopes to determine the next value of the variable. This method provides a more accurate approximation compared to Euler's method because it considers the changes in the derivative over the interval.
These rate constants are fundamental to the Hodgkin-Huxley model as they dictate the dynamics of the ion channels' opening and closing, which in turn control the flow of ions across the neuron's membrane and thus the generation and propagation of action potentials. The model's ability to replicate the behavior of neurons hinges on the accuracy of these functions in describing the voltage-dependence of ion channel kinetics.
The Hodgkin-Huxley model provides a quantitative description of the electrical characteristics of excitable cells such as neurons. It has been a cornerstone in the field of computational neuroscience and has been used extensively to study the mechanisms of action potential generation and propagation.
The FitzHugh-Nagumo Model
The FitzHugh-Nagumo model is a simplified version of the Hodgkin-Huxley model that describes the spiking activity of neurons. It reduces the complexity of the Hodgkin-Huxley model while retaining the essential features of action potential generation. The FitzHugh-Nagumo model consists of two differential equations that describe the dynamics of the membrane potential and a recovery variable. Here is the mathematical notation for the FitzHugh-Nagumo model:
Let V (t) represent the membrane potential of the neuron at time t , and w(t) represent the recovery variable. The FitzHugh-Nagumo model is given by:
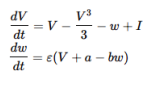
Where:
• V is the membrane potential.
• w is the recovery variable, representing processes such as ion channel recovery that contribute to the refractory period of the neuron.
• I is the external current or stimulus applied to the neuron.
• ε, a, and b are constants that determine the dynamics of the model. ε is a small parameter that controls the timescale separation between the fast dynamics of V and the slower dynamics of w . a and b are parameters that set the properties of the neuron, such as the threshold for firing and the reset properties.
The first equation describes how the membrane potential V changes over time, incorporating a cubic nonlinearity that captures the excitable nature of the neuron. The second equation describes the evolution of the recovery variable w, which provides feedback to the membrane potential and governs the refractory behavior of the neuron.
The FitzHugh-Nagumo model is widely used in computational neuroscience and mathematical biology to study neural excitability, action potential generation, and the dynamics of neural networks. Despite its simplicity, the model captures the essential features of neuronal spiking and refractory behavior, making it a valuable tool for theoretical and computational investigations.
Gradient Potential Neurons
The operation of neurons that use graded potentials, such as photoreceptors, bipolar cells, and horizontal cells in the retina, can be described mathematically in terms of graded response to stimuli rather than the all-or-nothing spiking behavior of action potential neurons. Graded potential neurons modulate their neurotransmitter release based on the degree of their membrane potential changes. The response of a graded potential neuron can be modeled using a continuous function that represents the relationship between the input signal and the neuron's membrane potential.
Let's denote the membrane potential of a graded potential neuron as V (t) at time t. The change in membrane potential in response to an input stimulus can be represented as follows:
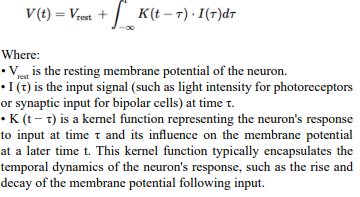
In this model, the graded potential of the neuron is computed as the convolution of the input signal with the kernel function over time. The resulting membrane potential V (t) determines the level of neurotransmitter release, which is proportional to the deviation of V (t) from the resting potential.
It is important to note that this representation is a simplification and idealization of the complex dynamics of graded potential neurons. In reality, these dynamics can be influenced by various factors, including receptor properties, synaptic integration, and cellular mechanisms like ion channel kinetics. Nonetheless, this model provides a basic mathematical framework for understanding how graded potential neurons process and integrate incoming signals.
Exceptional Neurons in The Processing Pathway
It is useful to understand the exceptional neurons to identify how the brain evolved to process image streams.
The visual processing pathway includes several types of neurons, each with specific roles in processing visual information. Among these, some neuron types are particularly unusual due to their unique structures, functions, or locations. Here are some of the most unusual neuron types in the visual processing pathway:
Retinal Ganglion Cells (RGCs) — Intrinsically Photosensitive RGCs (ipRGCs): ipRGCs are a unique subtype of RGCs that contain the photopigment melanopsin. Unlike other RGCs, which receive input from rods and cones, ipRGCs are directly sensitive to light. They play a crucial role in non-image-forming visual functions, such as circadian rhythm regulation and pupillary light reflex. The existence of RGCs that are directly photosensitive is a relatively recent discovery and represents an unusual adaptation in the retina.
Direction-Selective Ganglion Cells: These RGCs are specialized in detecting the direction of motion. They respond preferentially to movement in specific directions, which is crucial for motion detection and tracking moving objects. The mechanism for direction selectivity involves complex interactions with bipolar cells and starburst amacrine cells in the retina.
Starburst Amacrine Cells: Starburst amacrine cells have a distinctive radial, starburst-like dendritic morphology. They play a crucial role in motion detection and are integral to the circuitry that enables direction-selective ganglion cells to function. These cells release neurotransmitters in a directionally asymmetric manner, which is key to their role in motion detection.
Horizontal Cells: Horizontal cells exhibit wide-reaching dendrites that enable them to integrate signals across a large area of the retina. They are involved in lateral inhibition, a process that sharpens and enhances visual information. Their role in contrast enhancement and color processing, particularly their involvement in the antagonistic center-surround organization of photoreceptor receptive fields, makes them unusual and crucial for visual processing.
Double-Opponent Cells in V1: These cells in the primary visual cortex (V1) are involved in color processing. They have a unique receptive field organization where they respond to color contrast rather than uniform color. Double opponent cells are sensitive to specific color combinations in adjacent areas of their receptive fields, making them particularly important for detecting color edges and processing color within complex visual scenes.
Hypercomplex Cells (End-Stopped Cells) in V1: Hypercomplex cells, also known as end-stopped cells, are a type of neuron in V1 that respond to specific orientations and lengths of visual stimuli. They are similar to complex cells in their orientation selectivity but have the added feature of being sensitive to the length of a stimulus. These cells are thought to play a role in perceiving the ends of objects and detecting corners and angles, contributing to the interpretation of complex shapes and forms.
Mirror Neurons in Higher Visual Areas: Although not exclusively part of the primary visual processing pathway, mirror neurons, found in higher visual and cognitive areas, are unusual in that they fire both when an individual performs an action and when they observe the same action performed by another. Their presence in visual areas involved in processing observed actions highlights the complex interplay between visual perception and motor cognition.
Each of these neuron types exhibits unique properties and specializations that contribute to specific aspects of visual processing. From direct light sensitivity and motion detection to color processing and complex form perception, these unusual neurons underscore the complexity and sophistication of the visual system in extracting and interpreting a wide range of visual information.
History of Liquid State Machines
Liquid State Machines (LSMs) are a concept in the field of artificial intelligence and computational neuroscience, particularly within the area of spiking neural networks (SNNs). The history of LSMs is intertwined with the development of spiking neural networks and the exploration of more biologically realistic models of neural computation.
• Early Foundations in Neuroscience and AI: Before the concept of LSMs was formalized, there was significant research in neuroscience and AI on how the brain processes information. This research laid the groundwork for LSMs, as it involved understanding the dynamics of neural networks and the role of time in neural processing.
• Introduction of Spiking Neural Networks (SNNs): In the 1990s, there was a growing interest in SNNs, which are neural network models that more closely mimic the behavior of biological neurons compared to traditional artificial neural networks. SNNs operate based on spikes or discrete events, which are more representative of how real neurons communicate.
• Formalization of Liquid State Machines: The concept of LSMs was introduced by Wolfgang Mass and his colleagues in the early 2000s. LSMs are a class of revolt age neural network models designed to process time-varying inputs. They consist of a "liquid" — a randomly connected, spiking neural network that acts as a dynamic reservoir — and a "read-out" mechanism, which interprets the state of the liquid to produce an output.
• Biological Plausibility and Computational Efficiency: LSMs were appealing because they offered a framework that was more biologically plausible than traditional artificial neural networks and because they were computationally efficient in dealing with time-dependent signals. This made them particularly suitable for tasks such as speech recognition, motor control, and other tasks that involve time-series data.
• Research and Applications: Since their introduction, LSMs have been the subject of considerable research. They have been applied in various fields, including robotics, signal processing, and computational neuroscience. Research has focused on understanding the dynamics of LSMs, optimizing their architecture, and applying them to solve complex problems.
• Trends and Future Directions: Today, LSMs continue to be an active area of research, with ongoing efforts to improve their performance and understand their theoretical foundations. They are part of a broader trend towards more biologically inspired AI models and play a role in the intersection between AI and neuroscience.
LSMs represent an important step in the evolution of neural network models, moving towards systems that more closely resemble the dynamics of biological neural networks. Their development reflects a broader trend in AI and computational neuroscience towards models that are not only powerful in terms of computational capabilities but also offer insights into how the brain processes information.
The development of Liquid State Machines (LSMs) in AI has been marked by significant advancements and diverse applications. Here's a brief history and a bibliography of key papers:
• Initial Concepts and Applications: The LSM emerged as a computational model more adept than the Turing machine for computations in biological networks of neurons, introduced by demonstrated its application in speech recognition, showcasing its potential in pattern classification and function approximation using the inherent dynamics of the system [1,2].
• Theoretical Advancements: Contributed to the computational capability of LSMs by introducing a model with self-organized recurrent spiking neural networks (RSNN) and spike-timingdependent plasticity (STDP) [3]. Explored topological constraints and robustness in LSMs, highlighting the model's vulnerability to component failures and proposing solutions [4].
• Innovations in LSM Architecture: Presented a novel Parallelized LSM (PLSM) architecture for unintentional action detection, offering a lighter alternative to traditional deep-learning models [5]. Improved LSMs through iterative refinement of the reservoir, developing a method for refining randomly generated networks to yield more effective filters.
• Exploration of New Domains: Analyzed liquid ensembles for enhancing performance and accuracy in LSMs, proposing an ensemble of locally connected neuron reservoirs to improve latency and accuracy in speech and image recognition tasks [6].
• Advances in Machine Learning and AI: reviewed reservoir computing approaches to RNN training, providing insights into generating/adapting reservoirs and training different types of readouts in the context of LSMs [7]. Proposed the Deep Liquid State Machine (D-LSM), which explores recurrent spiking networks and deep architectures for computer vision applications.
• Hardware Implementations and Real-Time Applications: discussed the digital design of LSMs for real-time processing of spatiotemporal data, demonstrating its utility in EEG seizure detection and user identification based on walking patterns [8].
This bibliography provides an overview of the historical development and advancements in LSM technology in AI, highlighting key theoretical, architectural, and practical innovations.
Advantages of Spike-Based Processing
The use of spike-based neurons (spiking neural networks, SNNs) versus amplitude-based neurons (such as those in traditional artificial neural networks, ANNs) offers several advantages, particularly in terms of biological plausibility, computational efficiency, and suitability for certain types of tasks. Here are some of the key advantages of spike-based neurons:
• Biological Plausibility: Spiking neurons more closely mimic the behavior of biological neurons compared to amplitude-based neurons. Biological neurons communicate primarily through spikes (action potentials), which are all-or-nothing events. This spiking mechanism is fundamental to how the brain processes information, and spiking neural networks aim to replicate this aspect of neural computation.
• Temporal Dynamics: Spike-based neurons can capture the temporal dynamics of information processing. They can encode information not just in the rate of firing (as in rate-coded ANNs) but also in the precise timing of spikes [9,10]. This temporal coding allows SNNs to process time-dependent data more naturally and can be crucial for tasks that involve dynamic patterns, such as speech recognition or processing time series data.
• Energy Efficiency: In hardware implementations, particularly neuromorphic computing, SNNs can be significantly more energyefficient than traditional ANNs. This is because spiking neurons only need to process and transmit information when they fire, leading to sparse and event-driven computation. In contrast, amplitude-based neurons in ANNs typically involve continuous operations and data transmission, which can be more energyintensive.
• Suitability for Real-Time Processing: The event-driven nature of spiking neurons makes them well-suited for real-time processing tasks. They can react to inputs as they occur, making them ideal for applications that require quick and adaptive responses, such as robotics, sensor networks, and real-time data analysis.
• Information Encoding: Spike-based neurons can encode information in multiple ways, such as the rate of firing, the timing, or pattern of spikes, and the synchronization between neurons. This rich encoding capacity can potentially lead to more powerful and efficient representations of information compared to the amplitude-based encoding used in traditional ANNs.
• Learning Efficiency: Some learning algorithms for SNNs, such as spike-timing-dependent plasticity (STDP), are inspired by biological learning mechanisms. These algorithms can be more efficient in certain tasks, leveraging the temporal structure of spike trains for learning.
• Potential for New Computational Paradigms: The unique properties of spike-based neurons open up possibilities for new computational paradigms that are not easily achievable with traditional ANNs. For example, they are well-suited for implementing models of neural phenomena and exploring theories of brain function.
Firing Threshold
The firing threshold of a neuron is a fundamental concept in the study of neuronal behavior and signal processing. It represents a critical membrane potential level that a neuron must reach to generate an action potential, commonly known as a "spike." This threshold is pivotal in neuronal signaling and plays a significant role in how neurons process and transmit information [11-15].
At the heart of this process is the mechanism of triggering action potentials. The firing threshold is the specific level of membrane potential at which a neuron becomes active, leading to the firing of an action potential. This activation is typically the result of incoming stimuli that cause the neuron's membrane potential to depolarize. When this depolarization reaches the firing threshold, voltage-gated ion channels, particularly sodium (Na (^+)) channels, open rapidly. This opening results in a swift influx of Na (^+) ions into the neuron, causing a rapid and significant change in the membrane potential.
A key characteristic of action potential generation is its adherence to the all-or-none principle. According to this principle, an action potential is generated only if the membrane potential reaches or surpasses the firing threshold. If this threshold is not met, the neuron remains inactive, and no action potential occurs. This binary response ensures that neurons respond decisively to stimuli, either firing in full or not firing at all, based on whether the stimulus strength is sufficient to cross the threshold.
The firing threshold is also essential for the neuron's ability to encode information. Neurons can adjust their sensitivity to incoming stimuli by modulating their firing threshold. This modulation can be influenced by various factors, including the neuron's previous activity, the presence of neuromodulators, and the balance of excitatory and inhibitory synaptic inputs. Such adjustments allow neurons to adapt their responsiveness to changing conditions and to process information in a dynamic and context-dependent manner.
In addition to facilitating information encoding, the firing threshold acts as a control mechanism to prevent excessive or unwarranted firing. It serves as a filter, ensuring that only stimuli strong enough to induce a significant depolarization lead to action potentials. This filtering capability is crucial for maintaining the fidelity of neural signaling by preventing neurons from responding to minor or irrelevant stimuli.
The firing threshold is not a static value; it can vary between different neurons and can change over time within the same neuron. This dynamic adjustment of the threshold is influenced by the neuron's recent firing history and the nature of synaptic inputs it receives. For example, during periods of repetitive firing, certain ion channels may become activated, altering the neuron's membrane properties and effectively increasing the threshold. Conversely, certain synaptic inputs or neuromodulators can lower the threshold, making the neuron more likely to fire in response to subsequent inputs.
In neural computation, particularly in computational models of neurons, the firing threshold is a critical parameter. It determines when a neuron should output a signal, playing an essential role in models used in artificial neural networks, including spiking neural networks. In these models, the firing threshold dictates the pattern and timing of spikes, thereby influencing how information is processed and transmitted within the network.
Overall, the firing threshold is a key determinant of neuronal activity and plays a vital role in the complex processes of neural signaling and information processing. Its ability to dynamically adjust and respond to varying conditions underscores the adaptability and sophistication of neuronal function.
Firing Threshold
The firing threshold is essential for the neuron's ability to generate action potentials in response to specific inputs. It acts as a critical level that the membrane potential must reach for the neuron to become active, playing a fundamental role in the neuron's responsiveness and information processing capabilities.
The firing threshold at time t is calculated as:
Threshold (t) = Θbase + Nspikes (t) ⋅ ΔΘ
Where:
Threshold(t) is the firing threshold at time t.
Θbase is the base threshold for firing.
Nspikes is the number of spikes in the rolling window at time t.
ΔΘ is the amount by which the threshold increases per spike.
Threshold(t)
The concept of Threshold(t) in neuronal function encapsulates the dynamic and time-dependent nature of a neuron's firing threshold. Unlike a static or fixed threshold, Threshold(t) changes over time, adapting to the neuron's recent activity and the prevailing physiological and environmental conditions. This adaptability is crucial for the neuron's ability to modulate its excitability and responsiveness, ensuring effective neural signaling and processing.
One of the most significant aspects of is its time-dependent nature. This characteristic reflects the neuron's capacity to alter its firing behavior in response to ongoing patterns of stimulation. After periods of intense activity, for example, specific ion channels may become more or less active, or in some cases, inactivated. These changes can lead to an increase in the firing threshold, a phenomenon often referred to as spike frequency adaptation. This adaptation is critical in preventing excessive neuronal firing, and it plays a pivotal role in how neurons encode stimulus intensity and frequency.
Synaptic inputs also play a key role in modulating Threshold(t). The balance of excitatory and inhibitory inputs received by a neuron can significantly influence its firing threshold. Excitatory inputs tend to lower the threshold, making the neuron more prone to firing, while inhibitory inputs raise the threshold, reducing the likelihood of firing. This dynamic modulation is essential for the integration of synaptic inputs and for the regulation of neural circuits, ensuring that neurons respond appropriately to the complex array of signals they receive.
Neuromodulators, including various neurotransmitters and hormones, further influence Threshold(t). These substances can alter the properties of ion channels and receptors, leading to changes in the neuron's excitability and, consequently, its firing threshold. The modulation by neuromodulators is integral to a wide range of neural processes, encompassing areas such as learning, memory, and mood regulation.
In computational neuroscience, particularly in the development of spiking neural networks, Threshold(t) is a critical parameter. Accurately modeling this dynamic threshold is crucial for simulating realistic neuronal behavior. In these models, Threshold(t) determines when a neuron will generate an output signal, influencing how neural networks process and transmit information. Understanding and replicating this dynamic aspect of neuronal behavior is key to advancing our knowledge of neural computation and information processing in the brain.
Threshold(t) represents the evolving and adaptable nature of the neuronal firing threshold, highlighting the neuron's ability to fine-tune its responsiveness to stimuli over time [16-20]. This adaptability is fundamental to the complex information processing capabilities of the brain, enabling neurons to function effectively in a constantly changing environment. The dynamic threshold plays a crucial role in neural signaling, integrating synaptic inputs, and modulating neuronal excitability. It is also a key area of focus in both neuro-science research and computational modeling, providing insights into the intricate workings of neural circuits and the brain's remarkable ability to process information. Understanding Threshold(t) and its implications is essential for unraveling the mysteries of neural function and for developing more effective computational tools to simulate neural processes.
Base Threshold
The base threshold for firing, often simply referred to as the firing threshold, is a fundamental concept in neuroscience, particularly in the study of how neurons communicate and process information. This threshold represents the critical level of membrane potential that a neuron must reach to initiate an action potential, commonly known as a spike. Under-standing the base threshold for firing is essential for comprehending the complex mechanisms of neuronal signaling and the overall functioning of the nervous system
At its core, the base threshold for firing is the membrane potential level at which voltage-gated sodium channels are activated. These channels play a crucial role in the generation of action potentials. When the membrane potential of a neuron, due to incoming stimuli, depolarizes and reaches this threshold, a significant influx of sodium ions (Na (^+)) occurs. This influx causes a rapid and substantial change in the membrane potential, driving it towards a positive value [21-25]. The rapid depolarization that follows marks the beginning of the action potential, leading to the propagation of a signal along the neuron's axon.
The concept of the firing threshold follows the all-or-none principle, which is central to neuronal signaling. According to this principle, the neuron will fire an action potential only if the membrane potential reaches or exceeds the threshold. If the stimulus is insufficient and the threshold is not reached, the neuron remains inactive, and no action potential is generated. This binary response mechanism ensures that neurons do not respond to minor or insignificant stimuli, thereby maintaining the precision and specificity of neural communication.
The base threshold for firing is not a fixed value and can vary among different neurons. Even within the same neuron, the threshold can change over time, influenced by various factors such as the neuron's previous activity, the balance of excitatory and inhibitory synaptic inputs, and the presence of neuromodulators. These dynamic changes in the firing threshold allow neurons to adapt their responsiveness to the constantly changing internal and external environment [26,27]. For instance, during periods of high neuronal activity, certain ion channels may become activated, which can increase the threshold and reduce the neuron's excitability. This adaptive mechanism prevents excessive firing and helps regulate neural activity.
In addition to its role in individual neurons, the firing threshold has significant implications in neural networks and computational models of the brain. In artificial neural networks, including spiking neural networks, the firing threshold is a critical parameter that determines when a neuron should output a signal. It influences the pattern and timing of spikes within the network, affecting how information is processed and transmitted. Understanding and accurately modeling the firing threshold is therefore essential for developing effective computational tools and simulations of neural processes.
The base threshold for firing is a key determinant of neuronal activity, playing a pivotal role in the generation of action potentials and the transmission of signals in the nervous system [28-30]. Its dynamic nature allows for the adaptability and flexibility of neuronal responses, which is essential for the complex and varied tasks carried out by the brain. Understanding the mechanisms underlying the firing threshold is crucial for advancing our knowledge of neural function and for the development of computational models that accurately represent neural processes.
Number of Spikes per Window
Utilizing spikes in neurons to represent data offers a novel and biologically inspired approach to encoding and processing information, particularly when applied to different-sized windows for spike counting. This method provides a versatile means for data representation in neuromorphic computing and neural networks, where the neuronal activity is harnessed for computational purposes.
In the context of spike encoding, the size of the window used for counting spikes is pivotal in determining the level of precision and range of values that can be encoded. For instance, a window designed to count up to 1024 spikes aligns with 10-bit data representation, as 10 bits can encode 2(^10) (1024) distinct values. Similarly, a window accommodating up to 256 spikes corresponds to 8-bit data, offering 2(^8) (256) possible values. This alignment of spike count with binary data representation provides a direct and efficient method for encoding a wide range of values using neuronal spikes.
The dynamic range and precision of the encoded data are directly influenced by the window size. Larger windows enable the counting of more spikes, allowing for a broader range of values to be represented. This capability is particularly beneficial in applications requiring high precision or a large dynamic range, such as detailed image processing. In grayscale image representation, the number of spikes within a window can correspond to various shades of gray. A larger window, capable of representing 10-bit data, allows for a greater dynamic range, thus enabling the depiction of more nuanced and detailed grayscale images.
Furthermore, the choice of window size impacts the temporal precision of the spike-based encoding. Smaller windows offer higher temporal resolution, as they capture rapid changes in spike count, making them suitable for real-time processing and applications that demand quick responses. On the other hand, larger windows average out neuronal activity over a longer duration, providing a more stable and consistent representation, beneficial for reducing noise and ensuring reliability in the encoded data.
This spike-based approach to data representation has significant implications in the fields of neural networks and neuromorphic computing. Spiking neural networks (SNNs) can utilize different window sizes for spike counting to process and transmit information, closely mimicking the functionality of biological neural networks. Neuromorphic computing systems, which aim to replicate the structure and operation of the human brain, can leverage this method for efficient and biologically plausible information processing.
Threshold Increases per Spike
The parameter ΔΘ, representing the amount by which the firing threshold increases per spike, is a critical component in the adaptive mechanisms of neuronal function. This parameter encapsulates how neurons dynamically adjust their responsiveness to stimuli, an essential process for maintaining the balance and efficacy of neural signaling. The concept of ΔΘ is particularly relevant in understanding the phenomenon of neuronal adaptation, where the firing threshold of a neuron is not static but can change based on the neuron's recent activity.
At its core, ΔΘ signifies the incremental increase in the neuron's firing threshold that occurs with each action potential or spike. This mechanism ensures that after a neuron fires, it becomes temporarily less sensitive to subsequent stimuli. The degree of this desensitization is quantified by ΔΘ . A larger value of ΔΘ means that each spike leads to a more substantial increase in the threshold, making the neuron less likely to fire again immediately.
Conversely, a smaller value of ΔΘ results in a more modest increase in the threshold, allowing the neuron to remain more responsive to new stimuli.
The role of ΔΘ in neuronal adaptation is crucial for several reasons. Firstly, it serves as a protective mechanism to prevent neurons from becoming overstimulated or excessively active. This is particularly important in high-frequency firing scenarios, where the risk of neuronal fatigue or damage might be higher. By increasing the firing threshold, ΔΘ helps to regulate the neuron's activity, ensuring that it only responds to stronger or more significant stimuli after a spike.
Secondly, the adaptive increase in the firing threshold mediated by ΔΘ plays a vital role in neural coding and information processing. By modulating the neuron's sensitivity based on recent activity, ΔΘ allows the neuron to dynamically adjust its response to ongoing stimuli. This adaptability is essential for the complex tasks carried out by neural circuits, such as sensory processing, learning, and memory formation. For example, in sensory systems, ΔΘ can help neurons adapt to constant or repetitive stimuli, allowing them to become more attuned to changes or new information in the environment.
Furthermore, ΔΘ has significant implications for computational models of neural networks. In these models, incorporating a dynamic threshold adjustment mechanism can lead to more biologically realistic simulations of neural behavior. By adjusting the firing threshold based on recent spiking activity, these models can better replicate the adaptive responses observed in biological neurons. This capability enhances the models' ability to process information in a manner akin to the human brain, potentially leading to advancements in artificial intelligence and machine learning.
In addition to its role in individual neuron function, ΔΘ also influences the behavior of neural networks as a whole. In a network context, the collective adaptation of neurons through threshold modulation can lead to emergent properties, such as pattern recognition, synchronization, and oscillatory behaviors. These network-level phenomena are critical for various cognitive and physiological functions.
ΔΘ , the amount by which the firing threshold increases per spike, is a fundamental aspect of neuronal adaptation. It serves as a key mechanism for regulating neuronal excitability, preventing overstimulation, and enabling dynamic responses to stimuli. The incorporation of ΔΘ in neural models provides a more accurate representation of neuronal function and offers insights into the complex processes of neural information processing and computation. Understanding and modeling this adaptive threshold mechanism are crucial for advancing our knowledge of the brain and developing more sophisticated neural network architectures.
Neuron Firing and Reset
If V (t) > Threshold (t), the neuron fires, and the membrane potential is reset:
V (t) = Reset Potential. We most frequently reset to 0, but other values may be used, especially if the addition of random noise is desirable.
Spike Count Mechanism
The spike count is updated based on the rolling window:
Spike Count = Number of spikes in the last rolling window size, W, typically expressed in milliseconds (or frames).
The spike count decays over time, when no spikes occur. This leads to:
Veffective = Nspikes × C
Where:
t represents the voltage time at which the spike count is being calculated
Spike(s) is a function that equals 1 if the spike occurs at time s and 0 otherwise
Veffective represents the effective voltage.
Nspikes is the spike count, indicating the number of spikes in the time window
C is the factor used to convert spike count to voltage
The model incorporates membrane potential dynamics, adaptive threshold based on recent spiking activity, and a mechanism to calculate an effective voltage based on the spike count. The rolling window approach for counting spikes ensures that the neuron's adaptation is influenced by recent activity, providing a dynamic representation of the neuron's responsiveness over time.
Integration Back to Voltage
To integrate spikes over the time span from n to n + 1 , we consider the spike count adaptation mechanism.The spike count Spike Count(t) is the number of spikes that occur within a specified rolling window of size W ending at time t. The spike count can be expressed as an integral over the time window:
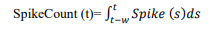
Where:
•∫tt −W t indicates that the integration (summation) is performed over the time interval from t −W to t , where t −W represents the start of the rolling window, and t represents the voltage time or the end of the rolling window.
• s is used as the variable of integration to represent different points in time within this window. s represents various points in time within the rolling window leading up to t. Using s allows us to differentiate between the voltage time (t) and each specific moment in the past (s) within the rolling window.
• t represents the voltage time at which the spike count is being calculated.
• Spike(s) is a function that equals 1 if the spike occurs at time s and 0 otherwise.
• W is the window size in milliseconds or frames.
• ds represent a small interval of time within the integration range. The integration sums up the value of Spike(s) over each small-time interval ds within the rolling window.
The integration is performed over the time window from t −W to d.
Neuron Response Rates
When a neuron is subjected to an increase in voltage, such as through synaptic input or direct stimulation, its response involves a series of rapid processes that are not immediate but follow a swift and sequential pattern. The response of the neuron to voltage changes is contingent upon the characteristics of the voltage increase and the inherent properties of the neuron itself.
Initially, upon receiving an increased voltage, the neuron undergoes depolarization of its membrane potential. The depolarization rate is influenced by the strength and duration of the voltage increase. Smaller or shorter voltage increases may lead to a more gradual depolarization, while larger or more prolonged increases can induce rapid depolarization. This change in membrane potential is a critical step in the neuron's response to voltage alterations.
A pivotal moment in the neuron's response is the potential generation of an action potential, which occurs if the depolarization reaches the neuron's firing threshold. When this threshold is surpassed, voltage-gated sodium (Na (^+)) channels open rapidly, allowing an influx of Na (^+) ions into the neuron. This influx further depolarizes the membrane and typically triggers an action potential if the threshold is sufficiently exceeded. The process from reaching the threshold to initiating an action potential unfolds within milliseconds, exemplifying the neuron's rapid response capabilities.
The neuron's response to increased voltage is characterized by the all-or-none principle in action potential generation [31-35]. This principle dictates that the neuron will either fire a full action potential (when the threshold is reached) or not fire at all (when the threshold is not reached). The action potential itself does not exhibit a gradual "ramp-up"; instead, it is a rapid and standardized response.
Following the firing of an action potential, the neuron enters a refractory period, which significantly in fluences its subsequent responsiveness. The absolute refractory period immediately follows the action potential and is a phase during which no new action potential can be generated, regardless of incoming stimuli strength. This period is succeeded by the relative refractory period, wherein a higher-than-normal threshold is necessary to elicit another action potential.
Moreover, the overall response of the neuron to increased voltage is also shaped by synaptic integration. Neurons often receive multiple synaptic inputs, integrated at their dendrites and cell body. The cumulative effect of these inputs, through temporal and spatial summation, can cause the membrane potential to reach the threshold more rapidly, thereby influencing the neuron's response time.
Accommodation
Accommodation or adaptation in neurons is a critical neurophysiological process where neurons exhibit a decrease in responsiveness to repeated or sustained stimulation. This phenomenon plays a pivotal role in modulating the neuron's excitability and preventing overstimulation, which is essential for the proper functioning and information processing within neural circuits.
One of the key mechanisms underlying neuronal accommodation is the inactivation of sodium channels. During the generation of action potentials, voltage-gated (Na+) channels are activated to allow an influx of (Na+) ions, leading to membrane depolarization. However, with repetitive neuronal activity, these channels can enter an inactivated state, where they become temporarily unable to open, even if the membrane depolarizes to the threshold level. This inactivation reduces the availability of (Na+) channels to initiate new action potentials, effectively raising the threshold required for firing and decreasing the neuron's excitability
Another important aspect of accommodation involves the activation of potassium (K+) channels, particularly calcium activated potassium channels. These channels are responsive to elevated intracellular calcium levels, which can occur as a result of high neuronal activity. The opening of these (K+) channels leads to an efflux of (K+) ions from the neuron, resulting in hyperpolarization or stabilization of the membrane potential at a more negative value. This hyperpolarization increases the difficulty for the neuron to reach the firing threshold, thus diminishing its responsiveness to subsequent stimuli.
The role of calcium (Ca2+) ions extends beyond the activation of potassium channels. Repetitive neuronal firing can lead to an accumulation of ions within the neuron, triggering various intracellular signaling pathways and influencing the properties of different ion channels. This modulation can significantly impact the neuron's excitability and responsiveness to external inputs.
Furthermore, the balance of excitatory and inhibitory synaptic inputs plays a substantial role in neuronal accommodation. An increase in inhibitory input or a decrease in excitatory input can contribute to the neuron's reduced responsiveness. Additionally, the release of neuromodulators in the brain can alter the properties of ion channels or synaptic receptors, further influencing neuronal excitability.
Implementation
GLSL
In our recent endeavor to advance the field of neural modeling, we have taken a significant step forward by implementing a new neuron design on the Graphics Processing Unit (GPU), utilizing the Graphics Shader Language (GLSL). This innovative approach capitalizes on the GPU's powerful capabilities for parallel computations, offering substantial improvements in computational efficiency and scalability, particularly beneficial for complex neural network simulations.
The decision to leverage the GPU for our neuron model is driven by its inherent strengths in parallel processing. Unlike traditional Central Processing Units (CPUs), which excel in sequential processing tasks, GPUs are adept at executing multiple operations simultaneously. This characteristic makes GPUs particularly wellsuited for simulating neural networks, where the computations of numerous neurons and their synaptic interactions can occur in parallel, greatly enhancing the processing speed.
Utilizing GLSL, a high-level shading language designed for programming GPUs, we have optimized our neuron model to harness the full potential of the GPU's architecture. GLSL enables precise control over GPU processing capabilities, allowing for efficient implementation of complex neural computations. These include the dynamic simulation of neuronal behavior, synaptic interactions, and network connectivity, all computed concurrently on the GPU.
The implementation of our neuron model on the GPU using GLSL offers several key advantages:
Firstly, the increased computational speed afforded by the GPU's parallel processing power is a gamechanger for neural simulations. This enhanced speed is critical for simulating large-scale neural networks and for applications that require real-time processing, such as neural prosthetics or brain-machine interfaces.
Secondly, the scalability of our model is greatly improved on the GPU platform. The GPU's capacity to handle a large number of calculations in parallel allows the model to scale effectively with network size. This scalability is essential for studying complex neural networks and for developing advanced artificial neural networks.
Furthermore, GPUs are generally more energy efficient than CPUs for parallel computations. This energy efficiency is particularly advantageous for reducing the power consumption of largescale simulations, making our model more sustainable and environmentally friendly
Finally, the computational power of the GPU enables us to incorporate more biologically realistic models of neuronal behavior into our simulations. This includes detailed modeling of synaptic dynamics, neuronal plasticity, and the diversity of neuron types. Such enhanced realism in simulations can be valuable for gaining more in-depth insights into neural dynamics and brain function.
VRAM Considerations
The utilization of Graphics Processing Units (GPUs) with large memory (VRAM) capacities represents a significant advancement in the field of neural modeling, especially when leveraging the Graphics Shader Language (GLSL) for implementation. The technical advantages offered by these high-capacity GPUs are manifold, particularly in the context of complex neural network simulations that demand substantial computational resources. One of the primary technical benefits of large VRAM GPUs is their ability to handle extensive data sets. Neural network simulations, especially those that aim to closely mimic biological neural systems, often involve a vast number of neurons and synapses. Each of these elements can have multiple attributes and states that need to be stored and processed. Large VRAM capacities enable the storage of detailed models of entire neural networks within the GPU's memory, facilitating efficient access and manipulation of this data during simulations. This is crucial for maintaining the fidelity and complexity of the network without compromising on performance.
Additionally, the parallel processing architecture inherent to GPUs is particularly well-suited for the simultaneous computation of numerous neuronal interactions. This capability is crucial for timebased processing tasks, such as dynamic neural network simulations and video analysis. The large VRAM al lows for more data to be processed in parallel, significantly enhancing the GPU's ability to handle real-time data analysis and complex computational tasks. This leads to improvements in processing speed and latency, which are essential for applications requiring rapid data processing and decision-making.
The increased memory capacity also facilitates the incorporation of more biologically realistic models in neural simulations. Advanced models often require the simulation of intricate synaptic dynamics, diverse neuron types, and complex neuronal plasticity mechanisms. Such models are data-intensive, as they involve detailed representations of various neural properties and interactions. Large VRAM GPUs provide the necessary computational space to accommodate these complex models, enabling more accurate and detailed simulations of neuronal behavior.
However, leveraging the full potential of large VRAM GPUs also involves certain technical challenges. Developing algorithms and software that can efficiently utilize the expanded memory and processing capabilities is critical. This requires an in-depth understanding of both neural modeling and GPU architecture, as well as expertise in high-performance computing and parallel programming.
Memory Requirements
To determine the memory requirements for a single neuron in a computational model and estimate how many such neurons would fit in 128 GB of Video RAM (VRAM), we need to consider various factors that contribute to the memory usage of each neuron. These factors include the size of variables representing the neuron's state, the number, and size of synaptic connections, and any additional data structures required for the neuron's operation.
Assuming a basic neuron model, the memory requirements might include:
• Neuron State Variables: Membrane potential, firing threshold, and other state variables. Assume each variable is a 32-bit floatingpoint number (4 bytes).
• Synaptic Weights: The number and size of synaptic weights if the neuron is part of a network. Each synaptic weight can also be a 32- bit floating point number.
• Additional Data Structures: Structures for synaptic inputs, outputs, and any auxiliary data required for neuron computation.
For a simple neuron model, let's assume the following:
• 10 state variables (e.g., membrane potential, threshold, etc.): 10 variables × 4 bytes = 40 bytes.
• 1000 synaptic weights (a rough estimate for a neuron with many connections): 1000 weights × 4 bytes = 4000 bytes.
• Additional data structures: Assume this takes up another 100 bytes.
Total memory per neuron ≈ 40 bytes + 4000 bytes + 100 bytes = 4140 bytes (approximately).
To calculate how many such neurons would fit in 128 GB of VRAM:
• 128 GB = 128 × 1024^3 bytes = 137,438,953,472 bytes.
• Number of neurons = Total VRAM / Memory per neuron = 137,438,953,472 bytes / 4140 bytes ≈ 33,192,533 neurons.
Therefore, approximately 33 million simple neurons could fit in 128 GB of VRAM.
It's important to note that this is a rough estimate. The actual number can vary significantly based on the complexity of the neuron model, the number of synaptic connections, and the specific implementation in GLSL. More complex neuron models with additional features, such as detailed ion channel dynamics or complex synaptic models, would require more memory per neuron, reducing the total number of neurons that could fit in the VRAM. Additionally, the efficiency of the GLSL implementation and the memory management strategy used will also influence the total capacity.
References
1. Maass, W. (2010). Liquid state machines: motivation, theory, and applications. Computability in context: computation and logic in the real world, 275-296.
2. Verstraeten, D., Schrauwen, B., Stroobandt, D., & Van Campenhout, J. (2005). Isolated word recognition with the liquid state machine: a case study. Information Processing Letters, 95(6), 521-528.
3. Xue, F., Hou, Z., & Li, X. (2013). Computational capability of liquid state machines with spike-timing-dependent plasticity. Neurocomputing, 122, 324-329.
4. Hazan, H., & Manevitz, L. M. (2012). Topological constraints and robustness in liquid state machines. Expert Systems with Applications, 39(2), 1597-1606.
5. Manna, S., Das, D., Bhattacharya, S., Pal, U., & Chanda, S. (2021). Plsm: A parallelized liquid state machine for unintentional action detection. IEEE Transactions on Emerging Topics in Computing, 11(2), 474-484.”
6. Wijesinghe, P., Srinivasan, G., Panda, P., & Roy, K. (2019). Analysis of liquid ensembles for enhancing the performance and accuracy of liquid state machines. Frontiers in neuroscience, 13, 204.
7. LukoševiÄius, M., & Jaeger, H. (2009). Reservoir computing approaches to recurrent neural network training. Computer science review, 3(3), 127-149.
8. Polepalli, A., Soures, N., & Kudithipudi, D. (2016, October). Digital neuromorphic design of a liquid state machine for realtime processing. In 2016 IEEE International Conference on Rebooting Computing (ICRC) (pp. 1-8). IEEE.
9. Bar-Cohen, Y., & Breazeal, C. (2003). Biologically inspired intelligent robots. Smart Structures and Materials 2003: Electroactive Polymer Actuators and Devices (EAPAD), 5051, 14-20.
10. Beerhold, J. R., Jansen, M., & Eckmiller, R. (1990). Pulseprocessing neural net hardware with selectable topology and adaptive weights and delays. In 1990 IJCNN International Joint Conference on Neural Networks (pp. 569-574). IEEE.
11. Cevora, G. (2019). The relationship between Biological and Artificial Intelligence.
12. Cotter, N. E., & Mian, O. N. (1992). A pulsed neural network capable of universal approximation. IEEE transactions on neural networks, 3(2), 308-314.
13. Del Corso, D., Gregoretti, F., & Reyneri, L. M. (1993). An artificial neural system using coherent pulse width and edge modulations. In Proceedings of the Third International Conference on Microelectronics for Neural Networks (pp. 105- 114).
14. Eguchi, H., Furuta, T., Horiguchi, H., & Oteki, S. (1991). Neural network hardware with learning function using pulseâ? density modulation. Electronics and Communications in Japan (Part II: Electronics), 74(11), 66-75.
15. El-Masry, E. I., Yang, H. K., & Yakout, M. A. (1997). Implementations of artificial neural networks using currentmode pulse width modulation technique. IEEE transactions on neural networks, 8(3), 532-548.
16. Faraguna, U., Nelson, A., Vyazovskiy, V. V., Cirelli, C., & Tononi, G. (2010). Unilateral cortical spreading depression affects sleep need and induces molecular and electrophysiological signs of synaptic potentiation in vivo. Cerebral cortex, 20(12), 2939- 2947.
17. B Fowler, B., & El Gamal, A. (1994). Pulse-modulated analog neuron circuits. Journal of VLSI signal processing systems for signal, image and video technology, 8(1), 45-51. 18. Gestri, G. (1971). Pulse frequency modulation in neural systems: A random model. Biophysical Journal, 11(1), 98-109.
19. Hamilton, A., Murray, A. F., Reekie, H. M., & Tarassenko, L. (1991). Working analogue pulse-firing neural network chips. In VLSI for Artificial Intelligence and Neural Networks (pp. 225- 233). Boston, MA: Springer US.
20. Hikawa, H. (2003). A multilayer neural network with pulse position modulation. Systems and Computers in Japan, 34(13), 36-46.
21. Hirose, T., Asai, T., & Amemiya, Y. (2007). Pulsed neural networks consisting of single-flux-quantum spiking neurons. Physica C: Superconductivity and its applications, 463, 1072- 1075.
22. Hofmann, L., Ebert, M., Tass, P. A., & Hauptmann, C. (2011). Modified pulse shapes for effective neural stimulation. Frontiers in neuro engineering, 4, 9.
23. Judd, K. T., & Aihara, K. (1993). Pulse propagation networks: A neural network model that uses temporal coding by action potentials. Neural Networks, 6(2), 203-215.
24. Krid, M., Damak, A., & Masmoudi, D. S. (2009). Hardware implementation of a pulse mode neural network-based edge detection system. AEU-International Journal of Electronics and Communications, 63(10), 810-820
25. Petriu, E. M., Watanabe, K., & Yeap, T. H. (1996). Applications of random-pulse machine concept to neural network design. IEEE transactions on instrumentation and measurement, 45(2), 665-669.
26. Reyneri, L. M. (1995). A performance analysis of pulse stream neural and fuzzy computing systems. IEEE Transactions on Circuits and Systems II: Analog and Digital Signal Processing, 42(10), 642-660.
27. L. M. Reyneri. (2002). Analog Integrated Circuits and Signal Processing. vol. 30, no. 2, pp. 101–119.
28. Tomberg, J., & Kaski, K. (1991, June). An effective training method for fully digital pulse-density modulated neural network architecture. In 1991., IEEE International Sympoisum on Circuits and Systems (pp. 1497-1500). IEEE.
29. Tomberg, J., Ritoniemi, T., Kaski, K., & Tenhunen, H. (1989). Fully digital neural network implementation based on pulse density modulation. In 1989 Proceedings of the IEEE Custom Integrated Circuits Conference (pp. 12-7). IEEE.
30. Tomberg, J., Ritoniemi, T., Tenhunen, H., & Kaski, K. (1989, May). VLSI implementation of pulse density modulated neural network structure. In IEEE International Symposium on Circuits and Systems, (pp. 2104-2107). IEEE.
31. J. Tomberg and K. Kaski. (1991). “Some Ic implementations of artificial neural networks using synchronous pulsedensity modulation technique.” International Journal of Neural Systems, vol. 02, no. 01n02, pp. 101–114, Jan.
32. Valtonen, K., Kronander, T., & Ingemarsson, I. (1990). Pulse stream neural networks and reinforcement learning. In 1990 IJCNN International Joint Conference on Neural Networks (pp. 267-272). IEEE.
33. Kim, Y. C., & Shanblatt, M. A. (1995). Architecture and statistical model of a pulse-mode digital multilayer neural network. IEEE transactions on neural networks, 6(5), 1109- 1118.
34. Kim, Y. C., & Shanblatt, M. A. (1995). Random noise effects in pulse-mode digital multilayer neural networks. IEEE transactions on neural networks, 6(1), 220-229.
35. Zhuang, H., Low, K. S., & Yau, W. Y. (2007). A pulsed neural network with on-chip learning and its practical applications. IEEE Transactions on Industrial Electronics, 54(1), 34-42.


