Research Article - (2024) Volume 1, Issue 2
Physics of Superconductors Polycyclic Aromatic Hydrocarbons (PAH)
Received Date: Jul 13, 2024 / Accepted Date: Jul 31, 2024 / Published Date: Sep 10, 2024
Abstract
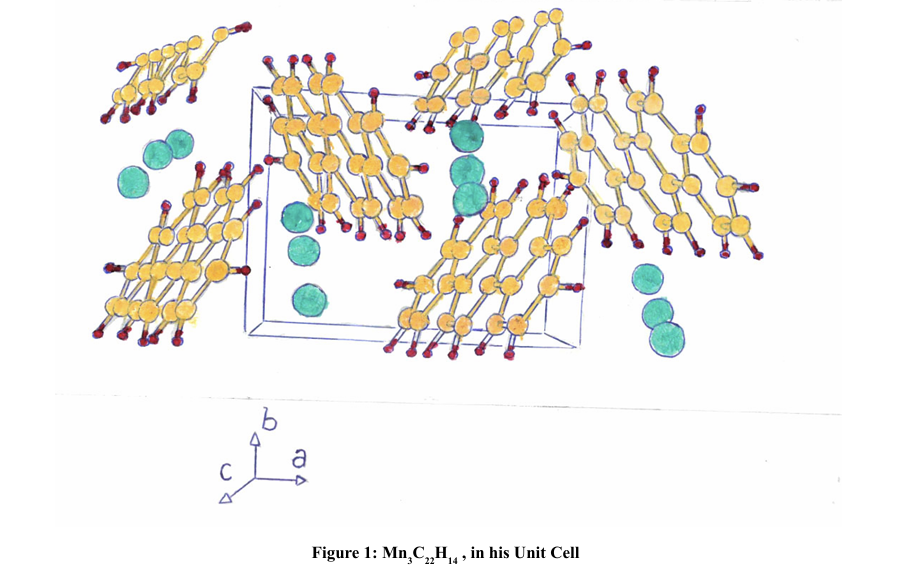
In this work, we will study metallic clusters equivalent to the organic ones intercalated with metals, of solid benzene type, Ba anthracene and K3.45picene; and we will try to calculate its superconducting Tc, for each of these compounds, from electronic correlations at room temperature. We will also study metal hydrides and clusters, totally composed of metals, which adopt forms analogous to molecular organic picene and anthracene; also calculating its TC.
Introduction
Solid hydrocarbon superconductors, consisting of benzene rings (C6H6), fused together, forming chains. When they form molecular solids, there are usually 2 molecules per unit cell. These solid hydrocarbons, doped with intercalated metals, were discovered in 2010 by Yoshiro Kubozono and his team [1,2]. They studied compounds, such as: k-picene (6.9 - 18°K), Rb3.8 picene, (11°K), Ca1.5 picene (7°K), Sm picene (4° K), K-penanthrene (4.95°K), Rb1penanthrene (4.75°K), Sr1.5 penanthrene (5.6°K), Ba1.5 penanthrene (5.4°K), Sm penanthrene (4.4 - 5°K), K3.45 dibenzopentacene (33°K), Sm chrysene (7.4°K), K3 coronero, (3.5 - 15°K), Ba anthracene (35°K), K3 pentacene (4.5°K) and K2tefernilo [3].
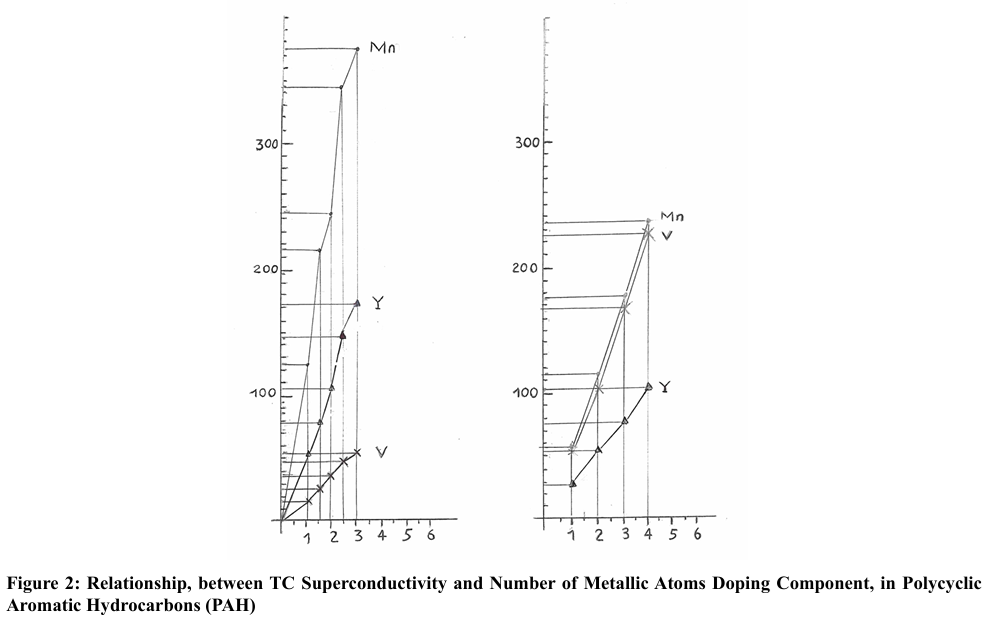
These molecular solids have a low superconducting fraction. The Ï? electrons in these molecules are delocalized; Because of this, these types of molecular solids behave like semiconductors. By inserting metals between the molecules, they transfer mobile electrons to said molecules, causing the solid to now behave like a metal, and can superconduct with a Tc higher than that of metal atoms when they form crystals [4]. However, during the last few years, some works that have been developed on the subject question the appearance of a superconducting phase, in this type of compounds, MXHAP. Some authors reported that they have found superconductivity in Ba anthracene (BaC14H10), with a Tc = 22°K; and in K picene (K3.45C22H14), with a Tc = 22°K. They attribute this superconductivity to a magnetic anomaly, which they identify with the magnetic susceptibility (Ï?m) [5]. In addition, these authors have not found a correlation between the number of benzene rings that form the chains, and the Tc, at which they superconduct; when they form molecular solid crystals doped with metals. The C and H atoms would not intervene in superconductivity in polycyclic aromatic hydrocarbons doped with metals.
Exposition
Relationship between Resistivity and Superconductivity
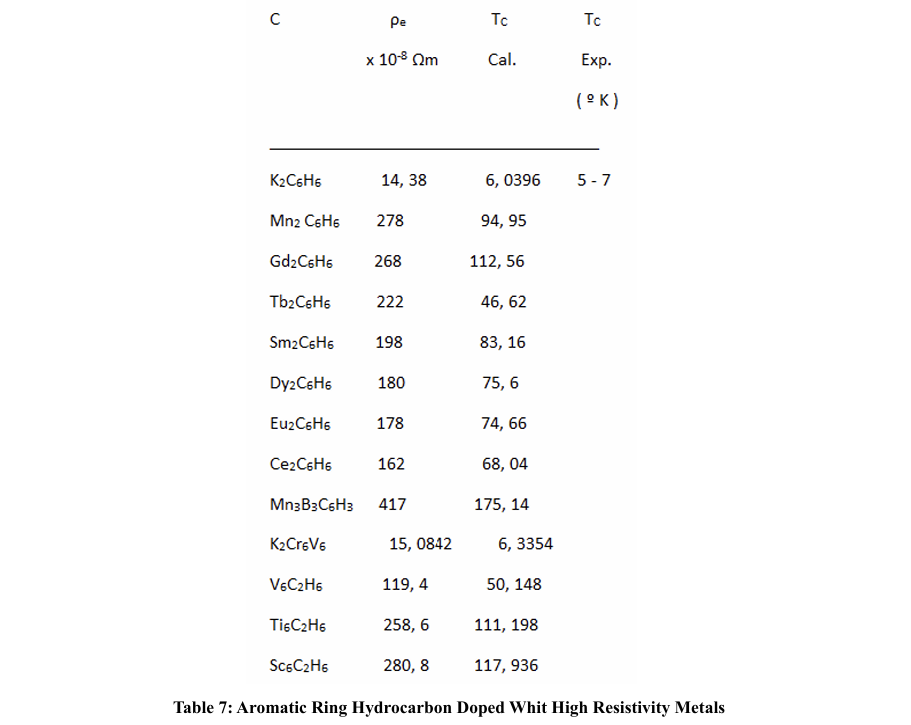
Minjae Kim, Hong Chul Choi, and other authors conclude in their study that superconductivity in solid polycyclic hydrocarbons doped with metals is of the conventional BCS type, mediated by phonons; instead of other more exotic mechanisms. In this line, a relationship can be established between the electronic resistivity and the superconducting Tc; that relates the interatomic distance and the intensity of the free electron - phonon coupling; this intensifying, at a smaller distance between atoms in the crystal lattice.
The electronic resistivity is defined by some parameters of previous calculation, for its determination. We first have its mass density:

where m, is the mass; and V is the volume of the unit cell. Then we have the density, or concentration of free electrons, in the solid.

In this expression, NA is Avogadro's number (6.02214 x 1023 mol-1); Z is the electronic valence number of the atom; A is the atomic mass number; and ρm, as already seen, in its previous expression, is the mass density. Per unit cell, this equation simplifies to: 
Being n°A, the number of atoms that make up the unit cell; which is what is used here.
The mean free path between collisions of the free electrons with the ions is defined by the equation.

Where the number of ions (n°ion) is directly proportional to their radius (rion); and inversely proportional to that of the mean free path (â??). Finally, we arrive at the final expression, for the electrical resistivity [7].
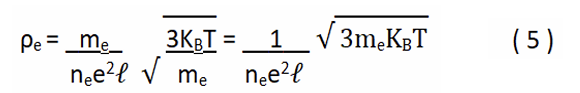
KB, is the Boltzmann constant (8.61733 × 10-5 eVK-1), and the temperature (T), comes in absolute degrees or Kelvin.
The electron's effective or dynamic máss, came difinited by the math formula:

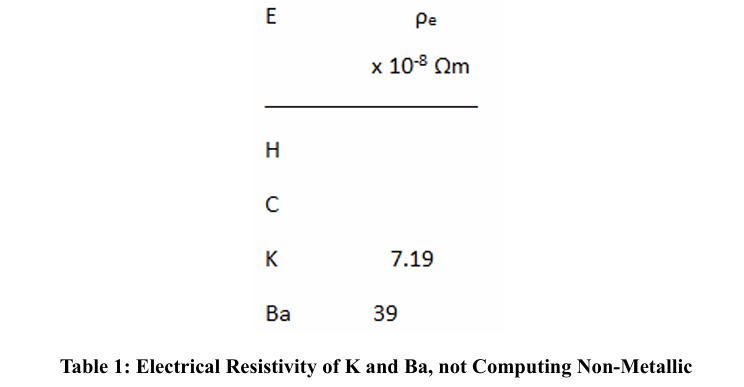
According to Kittel and G. T. Meaden, only metals compute electronic resistivity, excluding semi-metallic and non-metallic elements [9-11].
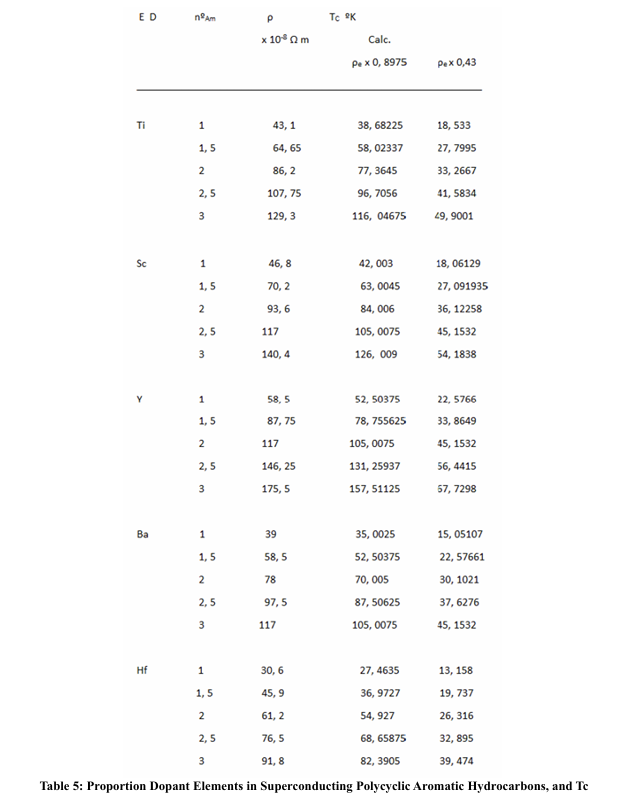
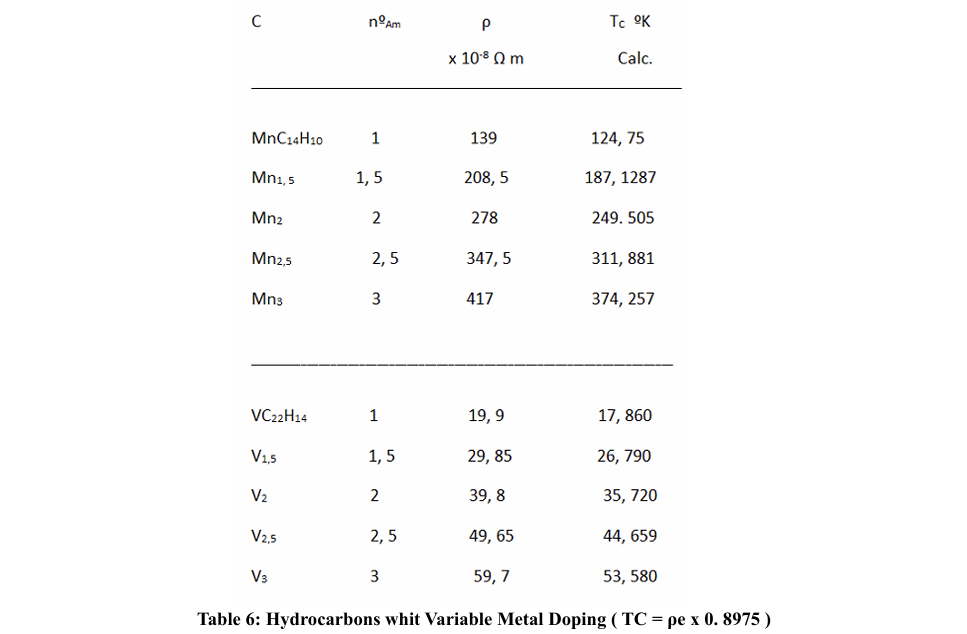
For the calculation of the superconducting TC, in the case of having only one metallic element (in this case, dopant metallic atoms), in a molecular solid, as is the case; we have found the relation:
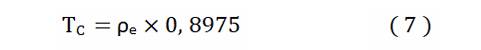
for Ba - anthracene and K - picene, is a fairly good approximation, for superconducting Tc.

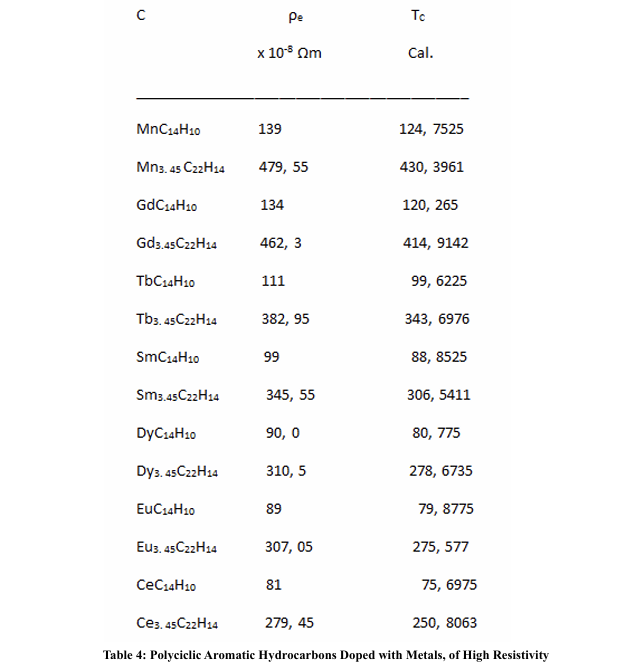
High Electrical Resistivity, among Metals
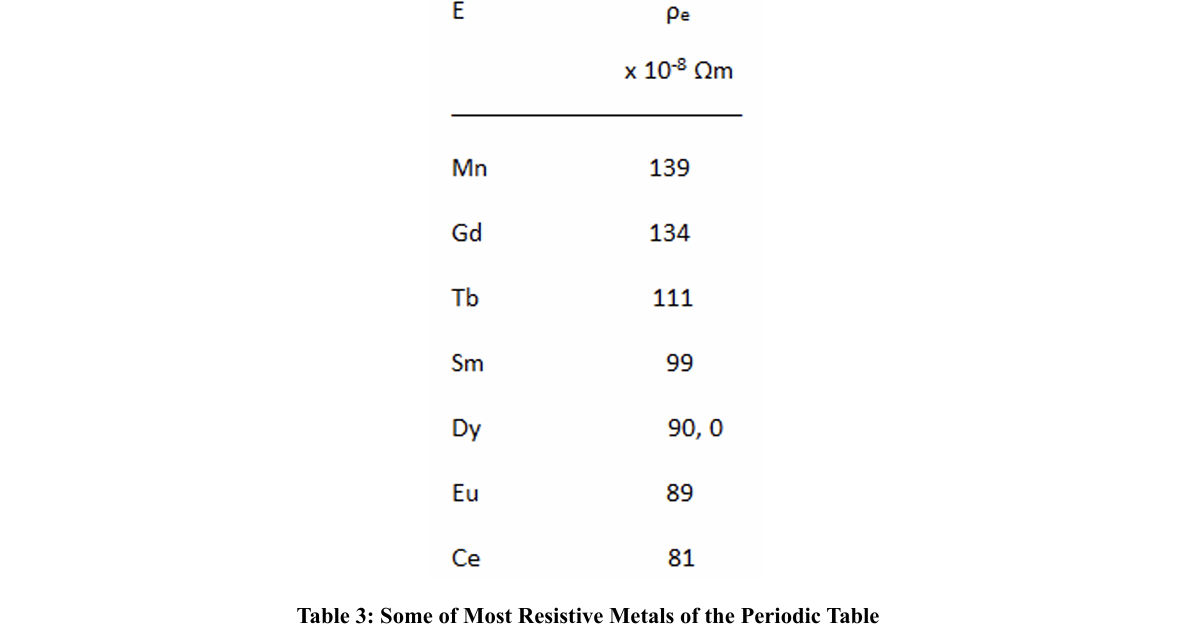
Some of the most resistive elements among the metals in the periodic table are:
For solids composed of simple or discrete molecules, of the solid benzene type, equation number 7 changes to:

When we have two components that form the star ring, such as Cr6V6, we can use the same equation that we used to calculate the reduced mass [11].
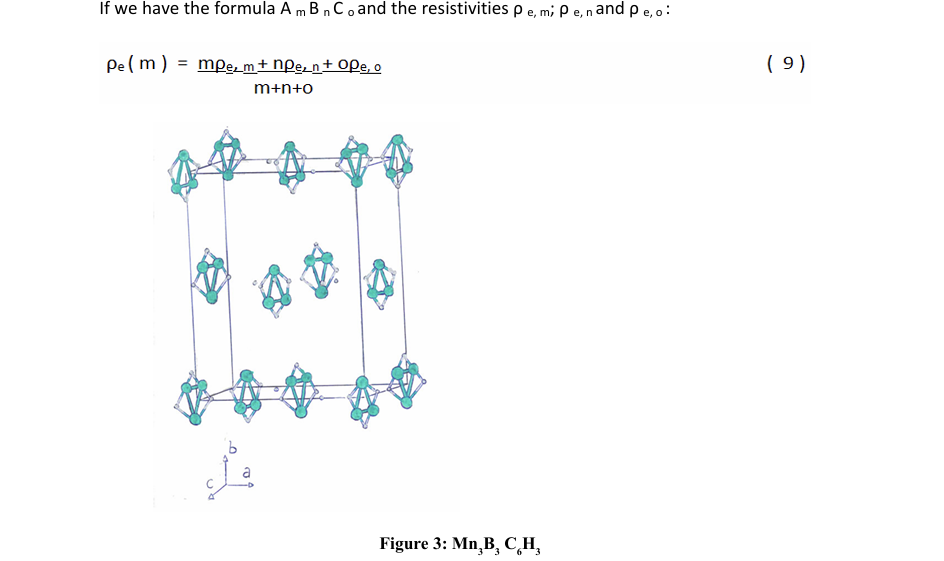
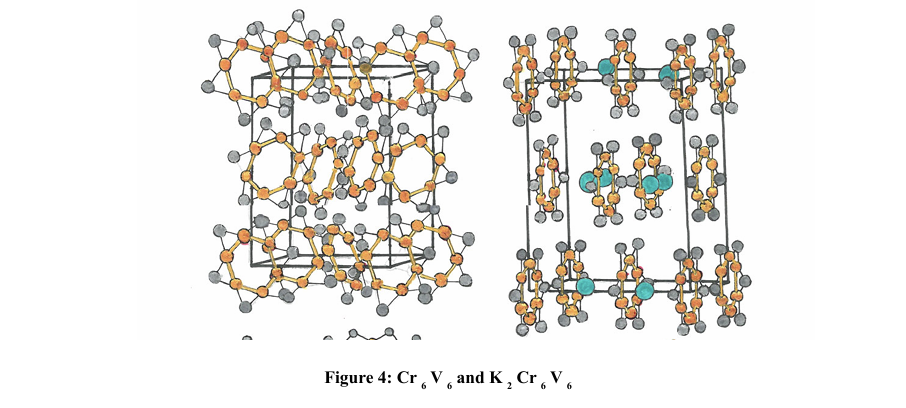
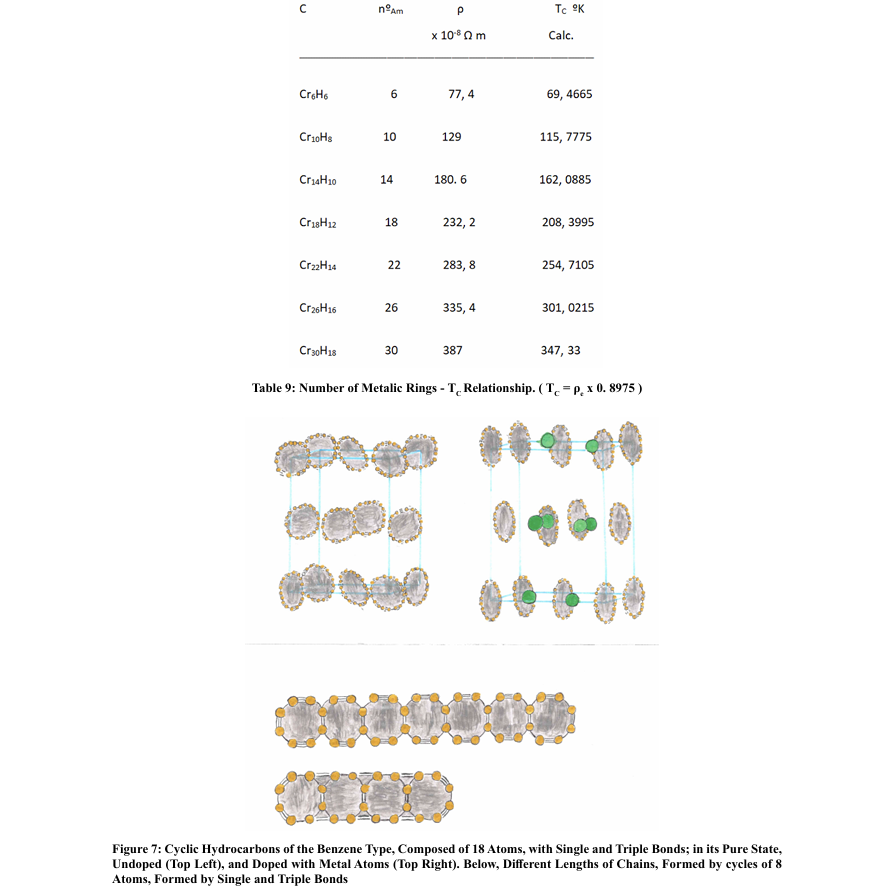
Conclusions
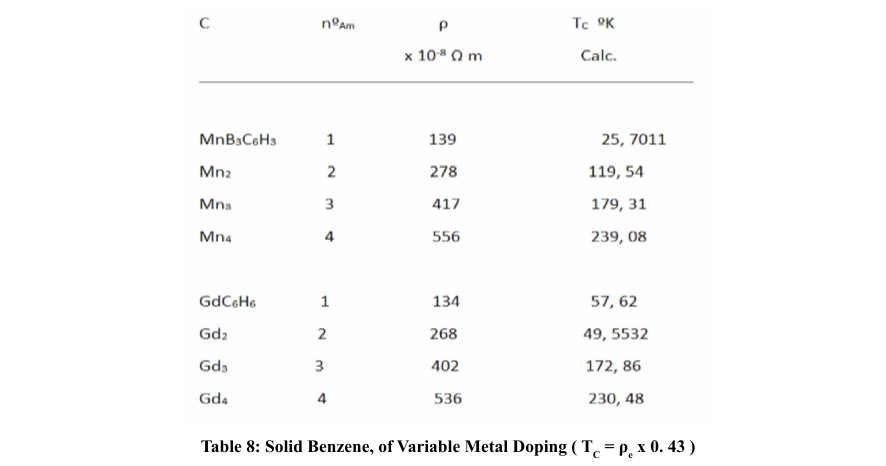
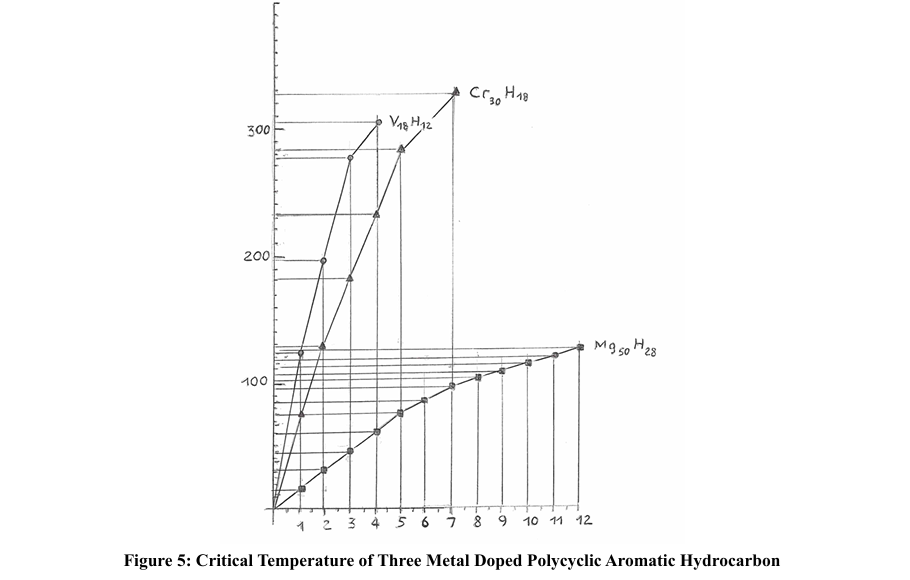
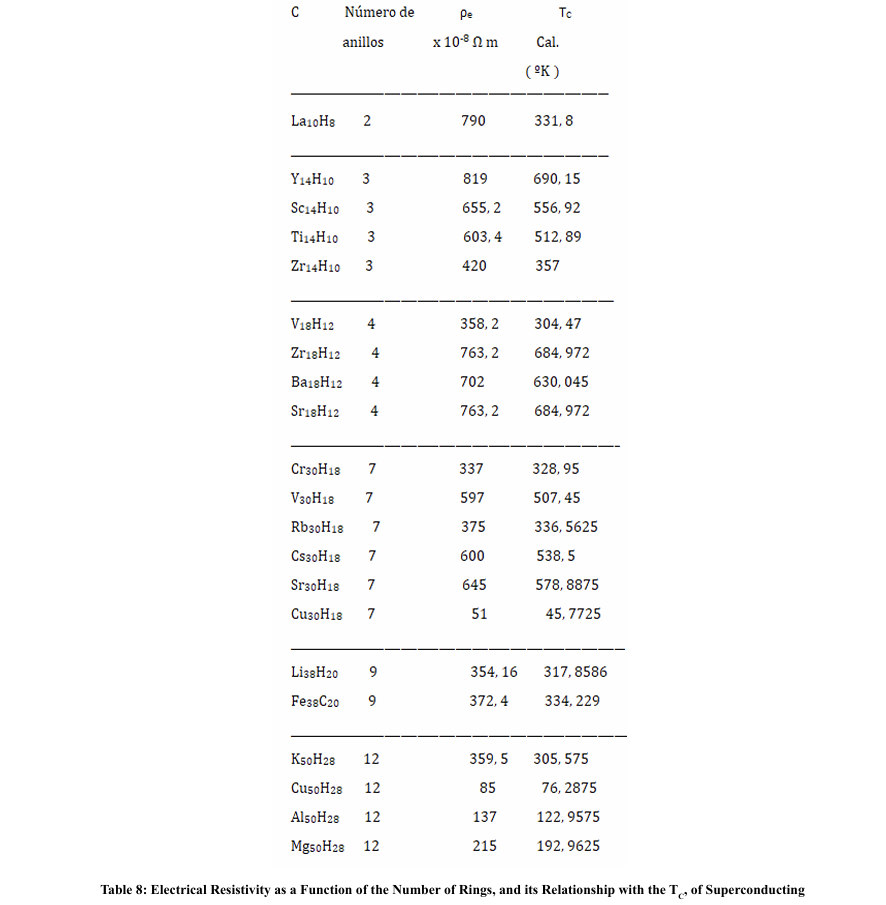
We have seen that when the dopant metals have a high resistivity, the TC seems to take higher values than when they have a low resistivity; likewise, when the rings are formed by metals with high electronic resistivity, when they are made up of two or three metals, the average resistivity is lower than when these rings are made up of only one kind of metal; the other components being non-metals or semi-metals. In any case, we do not have resistivity values per atom, which would imply not taking into account the collision times. On the other hand, if we could count on the effective mass, since this will depend on the density of electrons per atoms.
Author Contributions
The author contibuted to all this work.
Acknowledgments
This work has not received any type of support or funding of any kind. This work has been done on the initiative and curiosity of the author, for these topics of physics or chemistry-physics. I thank the internet communications channel for giving me access to specific documentation on this subject; as well as the authors reflected in the bibliography or reference, for their contribution, in the form of works, which have been consulted, for the realization of this study work.
Data Availability
The data concerning this manuscript has been deposited in a preprint service SSRN, belonging to the Sevier. It is also available on MDPI's preprint.org server. Its addresses are: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4891153
Author Declarations section
The authors have no conflicts to disclose.
References
1. Kubozono, Y., Goto, H., Jabuchi, T., Yokoya, T., Kambe, T., Sakai, Y., ... & Shimizu, K. (2015). Superconductivity in aromatic hydrocarbons. Physica C: Superconductivity and its applications, 514, 199-205.
2. Carrera, M. (2018). Materiales derivados de hidrocarburos aromáticos policíclicos intercalados con metales alcalinos.
3. Stefancic, A. (2017). Synthesis and characterization of alkali-metal reduced polycyclic aromatic hydrocarbon based materials (Doctoral dissertation, Durham University).
4. Slocombe, D. R., Kuznetsov, V. L., Grochala, W., Williams, R. J., & Edwards, P. P. (2015). Superconductivity in transition metals. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 373(2037), 20140476.
5. Hillesheim, D., Gofryk, K., & Sefat, A. S. (2015). On the nature of filamentary superconductivity in metal-doped hydrocarbon organic materials. Novel Superconducting Materials, 1(1).
6. Kim, M., Choi, H. C., Shim, J. H., & Min, B. I. (2013). Correlated electronic structures and the phase diagram of hydrocarbon-based superconductors. New Journal of Physics, 15(11), 113030.
7. Tipler-mosca-solucionary. Chapter 38.Solids and the Theory of Conduction. Page 2786, Vol-1.
8. Le Bahers, T., & Takanabe, K. (2019). Combined theoretical and experimental characterizations of semiconductors for photoelectrocatalytic applications. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 40, 212-233.
9. http://hyperphysics.phy-astr.gsu.edu/hbasees/Tables/ elecon.html
10. Kittel, Introduction to Solid State Physics, 7th Ed. Chapter 6 Free Electron Fermi Gas.
11. Meaden, G. T. (2013). Electrical resistance of metals. Springer.
12. Long-jian, L., & Zhuang-jian, Z. (2001). Investigation of empirical laws for superconductivity of alloy and compound superconductors. Chinese Physics, 10(9), 847.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



