Case Report - (2023) Volume 8, Issue 9
Mucoepidermoid Carcinoma Metastatic to Cervical Lymph Nodes with an Un-known Primary Site: A Rare Entity
2Department of Anatomical Pathology, National Institute of Oncology, University Mohammed V of Rabat, , Morocco
Received Date: Aug 08, 2023 / Accepted Date: Sep 01, 2023 / Published Date: Sep 20, 2023
Abstract
Mucoepidermoid carcinoma (MEC) is a malignant tumour that develops from the salivary glands; most commonly in the parotid gland, but may also involve the accessory salivary glands.
MEC represents both a diagnostic challenge, due to its high morphological variability, and a prognostic challenge due to a histological classification into three entities: high grade, low grade and intermediate grade.High-grade MEC can be very aggressive with a relatively high rate of regional and distant metastases.
Keywords
Mucoepidermoid carcinoma, Unknown primary, Cervical metastasis, Postoperative radiotherapy, Positron Emission Tomography (PET)/CT
Introduction
Mucoepidermoid carcinoma (MEC) is a malignant tumour that develops from the salivary glands; most commonly in the parotid gland, but may also involve the accessory salivary glands.
MEC represents both a diagnostic challenge, due to its high morphological variability, and a prognostic challenge due to a histological classification into three entities: high grade, low grade and intermediate grade.High-grade MEC can be very aggressive with a relatively high rate of regional and distant metastases.
Mucoepidermoid carcinoma metastatic to cervical lymph nodes with an unknown primary site is very rare, few cases have been described in the literature.The aim of this article is to describe the clinical and histological aspects and management of a nonprimary MEC through a case report.
Observation
We report a case of mucoepidermoid carcinoma metastatic to cervical lymph nodes with an unknown primary site in a chronic smoker and alcoholic 62-year-old patient. The initial symptomatology was a left cervical swelling, the cervical CT (computed tomography) scan showed a left sub-angulomaxillary Lymph node involvement of 25*30*45mm.
The left cervical lymph node curage had revealed several metastatic nodes of a Poorly differentiated squamous cell carcinoma. A PET scan was subsequently performed, showing active lymph node involvement laterocervical (Levels II and III) and left supraclavicular lymph node involvement (Figure 1).
A new work-up was performed with panendoscopy and biopsies,and an oeso-gastroduodenal fibroscopy Revenue all without any particularities.
The patient subsequently underwent a left radical curage involving the left jugular vein, which revealed lymph node metastases from a high-grade mucoepidermoid carcinoma with capsular rupture infiltrating the adventitia of the left jugular vein (Figure 2).
The patient received adjuvant radiotherapy at a dose of 66Gy to the left ganglionic areas (from the skull base down to the clavicle) and prophylactic radiotherapy to the homolateral parotid and submaxillary glands at a dose of 60Gy using the VMAT technique (Figure 3).
For planning radiation treatment, we use CT simulator (Siemens, Erlangen, Germany).
High-risk Clinical Target Volume (CTV-HR), include the anatomic sites at the highest risk of tumour invasion corresponding to the left ganglionic areas, and it was expanded by isotropic 5 mm margin to generate planning target volume (PTV). Organs at risk (cord, brainstem, optic chiasma, right eye, right lens, right retina, right optic nerve, temporal lobes, cochleae, right parotid gland, oral cavity, larynx,) were contoured.
Planning goals for the PTV based on ICRU 83 were the following: at least 90% and 95% of the prescribed total do (PTD) encompassing at least 98% and 95% of PTV, respectively (V≥98% and V≥ 95%, respectively); and no more than 2% of PTV received more than 107% of the PTV (V≤ 2%).
The following constraints were set for some OARs: for cord, the maximum dose received by 2% of its volume less than 45 Gy (D2%<45 Gy); for brainstem, D2%<55 Gy; for optic chiasm, D2%<60 Gy; for temporal lobes, D2%<65 Gy;for oral cavity Dmean<30-40Gy, and lastly, the mean dose received by right cochleae and left cochleae was respectively Dmean 25Gy and 23Gy (Figure 4).
Afterwards, the patient received external loco-regional radiotherapy at a dose of 50 Gy in 25 fractions, using the volumetric modulated arc therapy (VMAT) technique (Figure 3) plan with 6-MV photon beams was set by the medical physicist. VMAT plan was done with Elekta Versa HD. Patient position was verified weekly by kV cone beam CT imaging prior to treatment.
The patient tolerated the treatment and was seen weekly during treatment by our doctors.
No significant side effects were observed except mucite grade 1.
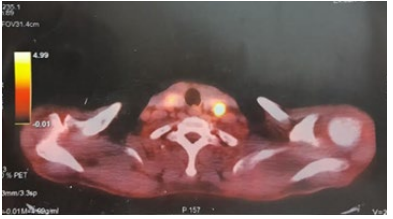
Figure 1: Axial positron emissiontomography (PET)/CT showing active lymph node involvement laterocervical (II and III) and left supraclavicular.
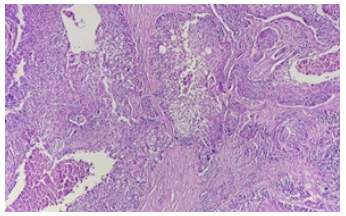
Figure 2: Solid and cystic areas of the tumor formed by different proportions of epidermoid (squamous) cells, mucus cells and intermediate cells (H&E stain, 10x).
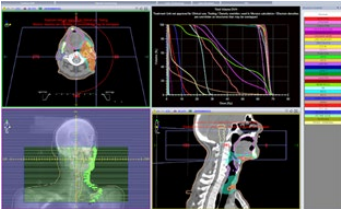
Figure 3: Computed tomography scan showing the target volumes: PTV-HR high risk in Red, PTH-IR intermediate risk in White. Volumetric modulated arc therapy was delivered to the region of high risk, to doses of 66 Gy.
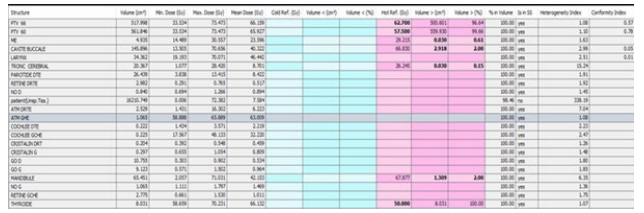
Figure 4: Dose-volume histogram distribution of the following structures: planning target volumes, spinal cord ; Oral cavity; larynx; brain stem; parotid glands (right); Right optic nerve; left optic nerve temporomandibular joints (right and left), right lens and left lens; ocular globes (right and left) cochleae(right and left); mandibular; thyroid gland.
Discussion
Salivary gland MEC is a rare malignancy. It accounts for 1-3% of malignant tumours of the head and neck and only 0.3% of all malignant tumours. It is characterised by epithelial proliferation of mucinous, intermediate and squamous cells [1].
The preferred site is, in decreasing order, the parotid gland (48%), the palate (20%) and the submandibular gland (7%) [2]. There is a slight female predominance. The average age of discovery varies between 40 and 60 years according to the authors [3]. Older age and the presence of lymph node metastasis with extraglandular invasion are factors of poor prognosis [4].
Its etiopathogenesis is not well understood and the clinical signs are generally not very suggestive, especially in the initial stages. To our knowledge, there has been 2 case report of MEC metastatic to the cervical lymph nodes in which the primary site was not identified [5].
MEC metastatic to the cervical lymph nodes in which the primary site typing is based on several histological criteria. Furthermore, according to Seethala, the histological grade is a significant indicator of the prognosis of MEC as it defines the degree of aggressiveness of the tumour and determines the management [6].
Poorer prognosis factors of MEC metastatic to the cervical lymph nodes in which the primary site are: age >40, male sex, high histologic grade; advanced stage, regional lymph node metastatic (rate of 72%); distant metastatic rate of 13% [7-10]. The management of MEC metastatic to the cervical lymph nodes in which the primary site depends on the pathological diagnosis. Surgical treatment remains the treatment of choice [6,17]. Lymph node dissection is indicated in high-grade tumours where the risk of lymph node metastases is greater than 50% [3-11].
Low-grade MEC are treated by surgery alone with good outcome and an estimated 5-year OS of >92%. Unlike high-grade MEC; which are at risk of local and distant recurrence with an estimated OS of 60% [9-10].
For inoperable patients or who refused surgery; 2 longterm studies on primary radiation therapy for salivary gland malignancies (patients receiving over 66 Gray (Gy).) showed 10-year local control rates of 57%14 and 75%,15 respectively, with a significant local control. Even if MEC has traditionally been considered radioresistant [12,13].
Adjuvant radiation increased locoregional control and improve overall survival for patients with positive Margins and advancedstage disease as well as for all high-grade tumors, where the risk of local recurrence and metastasis is high[10,11,14,16-19].
The toxicities of postoperative adjuvant radiation include facial dysfunction (epiphora and ectropion); osteoradionecrosis of the temporal bone and the jaw [20-21]. To our knowledge, there has been 2 case report of MEC metastatic to the cervical lymph nodes in which the primary site was not identified [28].
The first patient underwent a panendoscopy and incisional biopsy of a 6 cm neck mass that was positive for MEC. He was treated with an ipsilateral radical neck dissection and postoperative radiation from the skull base down to the clavicle [1].
The second patient was biopsied extensively at many common minor salivary gland sites, and underwent complete excision of the ipsilateral sublingual gland.
Parotid and submandibular glands were clinically and radiographically negative. Furthermore, PET scan was negative in identifying the primary site he underwent complete excision and adjuvant radiotherapy was recommended by a multidisciplinary tumor board. Our patient has not only had an unknown primary site but multiple risk factors for progression of disease (age, sex, stage, grade), thus adjuvant radiotherapy was recommended by a multidisciplinary tumor board.
Concomitant radiochemotherapy was not recommended for our patient.
Indeed, several series have evaluated the use of chemotherapy without salivary gland tumours with response rates ranging from 18% to 44% [23-25].
A Case control study based on retrospective medical record review comparing 2 arms of adjuvant therapy concomitant chemoradiotherapy and radiotherapy alone) in patients treated for locally advanced salivary tumours showed an improvement in overall survival with no difference in progression-free survival in favour of the concomitant chemoradiotherapy arm. However, the overall survival advantage was observed in patients with adenocarcinoma and salivary carcinoma; no difference was observed in the MEC subgroup Also high toxicity (grade 3) was observed in the concomitant chemoradiotherapy arm [21].
Retrospective studies evaluating the efficacy of PET/CT in detecting the extent of malignancy at the primary site show overall sensitivities ranging from 85.7% to 100% [26,27].
Our patient was treated with postoperative radiotherapy to the tumor bed, ipsilateral neck, and likely sites of an occult primary, such as the parotid and submandibular glands. The minor salivary glands were not included in the field given the negative examination under anesthesia. The patient completed radiation therapy and, on follow-up PET/CT 3months later, there was no uptake.
The long-term prognosis of mucoepidermoid carcinoma depends on healthy surgical margins and histological grade. Low-grade tumours have a 5-year survival of 90-100%, whereas this percentage falls to 20-40% for high-grade tumours [2,3].
Conclusion
The radiosensitivity of MEC is questionable, but in the case of MEC metastatic to cervical lymph nodes with an unknown primary site, irradiation of lymph node areas and suspected primary sites has shown significant benefit in controlling locoregional recurrence. However, the lack of randomised studies, particularly in regional lymph node metastases, does not allow its real effectiveness to be evaluated.
References
1. Spitz, M. R., & Batsakis, J. G. (1984). Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patients. Archives of Otolaryngology, 110(1), 45-49.
2. Baglin, A.C. (2015). Histoseminar on ENT tumour pathology, Carrefour pathologie 2004. Report of the French Society of Pathology. Paris, 2004. French Society of Pathology (page consulted on 18/06/2015). Espace multimédia. http:// www. sfpathol.org/histoseminaires-2004.html
3. da Cruz Perez, D. E., Pires, F. R., Lopes, M. A., de Almeida, O. P., & Kowalski, L. P. (2006). Adenoid cystic carcinoma and mucoepidermoid carcinoma of the maxillary sinus: report of a 44-year experience of 25 cases from a single institution. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons, 64(11), 1592–1597.
4. Hoyek-Gebeily, J., Nehmé, E., Aftimos, G., SaderGhorra, C., Sargi, Z., & Haddad, A. (2007). Cancer mucoépidermoïde des glandes salivaires: signification pronostique des marqueurs tumoraux [Mucoepidermoid carcinoma of salivary glands: the prognostic value of tumoral markers]. Revue de stomatologie et de chirurgie maxillo-faciale, 108(6), 482–488.
5. Prabhu, V., Johnston, J., Ingrams, D., & Passant, C. (2011). Mucoepidermoid carcinoma—Unknown primary and late distant metastasis: An unusual course of the disease. Clinics and Practice, 1(4), e97.
6. Seethala, R. R. (2009). An update on grading of salivary gland carcinomas. Head and neck pathology, 3, 69-77.
7. Guzzo, M., Andreola, S., Sirizzotti, G., & Cantu, G. (2002). Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Annals of surgical oncology, 9, 688-695.
8. Hicks, M. J., Elâ?Naggar, A. K., Flaitz, C. M., Luna, M. A., & Batsakis, J. G. (1995). Histocytologic grading of mucoepidermoid carcinoma of major salivary glands in prognosis and survival: a clinicopathologic and flow cytometric investigation. Head & neck, 17(2), 89-95.
9. Pires, F. R., De Almeida, O. P., De Araújo, V. C., & Kowalski, L. P. (2004). Prognostic factors in head and neck mucoepidermoid carcinoma. Archives of Otolaryngology– Head & Neck Surgery, 130(2), 174-180..
10. Ozawa, H., Tomita, T., Sakamoto, K., Tagawa, T., Fujii, R., Kanzaki, S., ... & Fujii, M. (2008). Mucoepidermoid carcinoma of the head and neck: clinical analysis of 43 patients. Japanese journal of clinical oncology, 38(6), 414- 418.
11. Aro, K., Leivo, I., & Mäkitie, A. A. (2008). Management and outcome of patients with mucoepidermoid carcinoma of major salivary gland origin: a single institution’s 30â?year experience. The Laryngoscope, 118(2), 258-262.
12. Chen, A. M., Bucci, M. K., Quivey, J. M., Garcia, J., Eisele, D. W., & Fu, K. K. (2006). Long-term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. International Journal of Radiation Oncology Biology Physics, 66(4), 1044-1050.
13. Mendenhall, W. M., Morris, C. G., Amdur, R. J., Werning, J. W., & Villaret, D. B. (2005). Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer, 103(12), 2544-2550.
14. Rapidis, A. D., Givalos, N., Gakiopoulou, H., Stavrianos, S. D., Faratzis, G., Lagogiannis, G. A., ... & Patsouris, E. (2007). Mucoepidermoid carcinoma of the salivary glands: Review of the literature and clinicopathological analysis of 18 patients. Oral oncology, 43(2), 130-136.
15. Hosokawa, Y., Shirato, H., Kagei, K., Hashimoto, S., Nishioka, T., Tei, K., ... & Nakamura, M. (1999). Role of radiotherapy for mucoepidermoid carcinoma of salivary gland. Oral oncology, 35(1), 105-111.
16. Armstrong, J. G., Harrison, L. B., Spiro, R. H., Fass, D. E., Strong, E. W., & Fuks, Z. Y. (1990). Malignant tumors of major salivary gland origin: a matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Archives of Otolaryngology–Head & Neck Surgery, 116(3), 290-293.
17. North, C. A., Lee, D. J., Piantadosi, S., Zahurak, M., & Johns, M. E. (1990). Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. International Journal of Radiation Oncology Biology Physics, 18(6), 1319-1326.
18. Triantafillidou, K., Dimitrakopoulos, J., Iordanidis, F., & Koufogiannis, D. (2006). Mucoepidermoid carcinoma of minor salivary glands: a clinical study of 16 cases and review of the literature. Oral diseases, 12(4), 364-370.
19. Brandwein, M. S., Ivanov, K., Wallace, D. I., Hille, J. J., Wang, B., Fahmy, A., & Mills, S. E. (2001). Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. The American journal of surgical pathology, 25(7), 835-845.
20. Mendenhall, W. M., Morris, C. G., Amdur, R. J., Werning, J. W., & Villaret, D. B. (2005). Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer, 103(12), 2544-2550.
21. Tanvetyanon, T., Qin, D., Padhya, T., McCaffrey, J., Zhu, W., Boulware, D.,. & Trotti, A. (2009). Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Archives of Otolaryngology–Head & Neck Surgery, 135(7), 687-692.
22. Trosman, S., Chute, D., Wood, B., & Lamarre, E. (2015). Unknown primary mucoepidermoid carcinoma: diagnosis and treatment. Head & Neck, 37(2), E22-E25.
23. Laurie, S. A., & Licitra, L. (2006). Systemic therapy in the palliative management of advanced salivary gland cancers. Journal of clinical oncology, 24(17), 2673-2678.
24. Triozzi, P. L., Brantley, A., Fisher, S., Boyce Cole, T., Crocker, I., & Huang, A. T. (1987). 5â?Fluorouracil, cyclophosphamide, and vincristine for adenoid cystic carcinoma of the head and neck. Cancer, 59(5), 887-890.
25. Jeong, H. S., Chung, M. K., Son, Y. I., Choi, J. Y., Kim, H. J., Ko, Y. H., & Baek, C. H. (2007). Role of 18F-FDG PET/CT in management of high-grade salivary gland malignancies. Journal of Nuclear Medicine, 48(8), 1237-1244.
26. Kim, M. J., Kim, J. S., Roh, J. L., Lee, J. H., Cho, K. J., Choi, S. H., & Kim, S. Y. (2013). Utility of 18 F-FDG PET/ CT for detecting neck metastasis in patients with salivary gland carcinomas: preoperative planning for necessity and extent of neck dissection. Annals of surgical oncology, 20, 899-905.
27. Trosman, S., Chute, D., Wood, B., & Lamarre, E. (2015). Unknown primary mucoepidermoid carcinoma: diagnosis and treatment. Head & Neck, 37(2), E22-E25.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



