Research Article - (2024) Volume 9, Issue 6
Histo-Morphological Assessment of the State of Microcirculation in Acute Experi-mental Pancreatitis
Received Date: Jun 08, 2024 / Accepted Date: Jun 17, 2024 / Published Date: Jun 25, 2024
Copyright: �©2024 Abbasova HF. This is an open-access article dis-tributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Citation: Abbasova, H.F. (2024). Histo-Morphological Assessment of the State of Microcirculation in Acute Experimental Pan-creatitis. J Clin Rev Case Rep, 9(6), 01-08.
Abstract
In order to study the features of hemolymphcirculation disorders in the pancreas that occur during acute pancreatitis (AP), morphological studies were carried out on experimental animals–48 rabbits in an intact state (8 rabbits) as well as 12, 24, 48 hours after modeling AP (10 rabbits in each series).
A model of AP was created by injecting into the pancreatic tissue of these animals a special complex, consisting of a mixture of bile with chloroform, at a dosage of 10 mg/100 g of weight under anesthesia after an upper laparotomy. In all series the morphostructure of the pancreas and its vascular microcirculatory (MC) system was studied.
It was detected that during the first day after modeling blockage of the background of a developing blockage of the microhemocirculaty (MHC)-link of microcirculatory MC system, simultaneously activated the drainage and transport functions of the microlymphcirculatory (MLC)-link of the MC system and the lymphatic system generally. On the third day a complete blockage of the MHC-link of the MC system occurs, while the MLC-link, despite the destruction of part of the lymphcapillaries, mostly continues to carry out its drainage and transport function. The blockage of the MHC-link of the MC system with the simultaneous activation of the drainage and transport function of the MLC-link of MC system and the lymphatic system, generally explains the low clinical effectiviness of conventional infusion-transfusional therapy for patients with AP and this is the rationale for the use of direct endolymphatic therapy.
Keywords
Acute pancreatitis, Regional microhemolymphcirculation
Actuality
Acute pancreatitis (AP) has been and remains one of the actual problems of emergency surgery. According to the World Health Organization (WHO), the morbidity by AP varies from 200 to 800 patients per 1 million population per year. In recent years, there has been a significant increase in the number of patients with AP, which steadily occupies the 1-2 places in the structure of acute surgical diseases of the abdominal organs, ahead of acute cholecystitis, acute intestinal obstruction, complicated gastric and duodenal ulcers acute appendicitis and strangulated hernia. In USA, the number of patients admitted to the clinic with this diagnosis exceeds 210.000 cases per year [8]. Their number is growing every year, and the annual cost of hospitalizations for patients with this pathology, only in USA is more than $2,5 billion [6]. In the structure of deaths from diseases of the abdominal organs, AP is in the first place, and the mortality rates for this diseases directly depend on its form: the edematous form gives a minimal mortality rate-no more than 10%, sterile pancreonecrosis-from 10 to 30%, and with an infected form it can reach-65% [2,3,7,8].
At present time it has been proven that acute pancreatic edema, pancreatic necrosis and purulent pancreatitis are phases of a single pathological process [1,5,9]. However, the severity of intraorgan hemocirculation disorders in the pancreas and the degree of participation of its lymphatic system in the development of each phase of the disease are not yet entirely clear, although the lymphatic system of the pancreas is the bed through which the products of the biochemical activity of the pancreas are carried into the thoracic duct and then into the blood [10-12]. Due to all of the above mentioned, experimental-clinical studies devoted to studying of the features of the state of intraorgan microcirculation (MC) of the pancreas are particularly relevant today.
Based on all the above mentioned, the main aim of our research was-to study the features of hemo-and lymph circulation disorders in the pancreas after creating a model of acute pancreatitis in experimental animals in dynamics at different periods of the inflammatory–destructive process.
Materials and Methods
Morphological methods for studying MC disorders were carried out in 48 rabbits in an intact state (8 rabbits), as well as 12,24,48 and 72 hours after modeling AP (10 rabbits in each series).
A model of AP was created at the research center of the Azerbaijan Medical University (AMU), by injecting into the pancreatic tissue of these animals under anesthesia a special complex consisting of a mixture of bile with chloroform (in a ratio of 2/1) at a dosage of 10 mg/100 g of weight, after an upper laparotomy.
The research was carried out in compliance with the rules stipulated by the European Commission for the supervision of laboratory and other experiments, involving experimental animals of different species. For histological studies, the pancreas and parapancreatic fatty tissues were taken. Fixation was carried out in 10% neutral formalin. After washing in running water, the specimens were processed through alcohols with increasing concentrations and embedded in paraffin with wax. Slices with a thickness of 5 mkm were prepared from paraffin blocks.
In all series, the morphostructure of the pancreas and its vascular (microcirculatory) system were studied. Changes in microhemoand lymphcirculation were traced by impregnation of the inner membrane of arterial, venous, lymphatic vessels and capillaries, with silver nitrate, according to V.V.Kupriyanov (1965). The material was painted with hematoxylin-eosin, according to the standard method and picro-fucsin mixture according to VanGieson. Methods of filling blood and lymphatic vessels with 30% solution of indigocarmine were also used (R.Lilly,1969).
It should be noted, that from several dozen micropreparations of slices various parts of the pancreas, for greater clarity, we selected preparations of those areas where changes in its vascular system are most demonstrative and prevail over changes in its glandular morphostructure.
Results and Discussions
The complex of methods used in the work made it possible to assess the structural state of the microvasculatory bed under normal conditions (Figure 1). On impregnated preparations, the arterial vessels of the pancreas have a winding passages, strongly marked tinctorial properties and pass into a dense net of precapillary blood vessels. Lymphatic vessels are not always accompanied by blood vessels; they have an autonomous type of distribution, but at the same time they also form a finely looped dense net of the drainage system.
Hemocapillaries are wide, winding; tightly distributed in composition of connective tissue base of the pancreas, densely anastomosied between each other. Their endothelial layer is well impregnated with silver nitrate, the cells have a polygonal shape, clear boundaries and standard sizes. The inner membrane of arterial and venous vessels is clearly impregnated with silver nitrate.
The studied area of the pancreas has an extremely well developed system of lymphatic vessels. Lymphcapillaries, in most cases form plexuses, merge in a dichotomous manner and form relatively large vascular formations. In diameter, lymphcapillaries are several times larger than similar structures of the hemodynamic bed, and have a more winding passage than hemocapillaries.
Through certain intervals, small thickenings or swelling are observed; the wall of the lymphcapillaries is less developed than the vascular wall of the hemocapillaries. Thus, hemocapillaries should be distinguished from lymphcapillaries by several morphological parameters: by tinctorial features, type of distribution and diameter of cross-section.
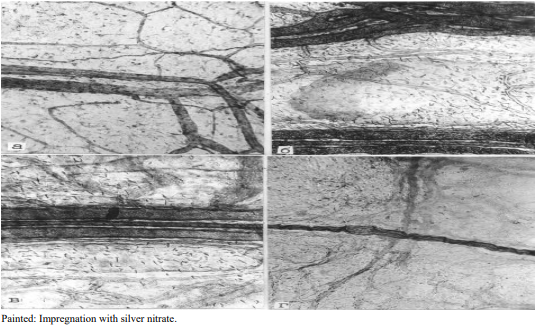
Figure 1: Norm. membrane preparation. A) Branching of hemocapillaries and formation of collecting venules; B) The collecting venule, a rteriole and group of lymphcapillaries form a microcirculatory vascular fascicle; C) System of lymphcapillaries connecting two microcirculatory fascicles–“vascular triads” in the pancreas; D) Net of lymphatic capillaries intersects with a nerve fiber that is well impregnated with silver nitrate according V.V.Kupriyanov
However, unlike the hemodynamic bed, separate lymphatic collectors almost always arise. They have form of a closed or half-enclosed system. Often several collectors merge together, form large lymphatic complexes, which apparently regulate lymphatic drainage from individual areas of the pancreas. Lymphatic vessels are formed as a result of the merger of several lymphacapillaries with the subsequent influx of lymph by the main or dichotomous type.
In experimental destructive pancreatitis, a whole complex of structural and functional changes unfolds in the pancreas of experimental animals, one of the manifestations is a disturbance of lymphohemodynamics in the lymphatic and blood vessels. Already 12 hours after pancreatitis modeling, a sharp swelling of mesothelial cells, blurring of their cell boundaries and pronounced interstitial edema are observed. There is complex of dystrophic disorders of interstitial intercellular spaces. This is especially clearly manifested when preparations are impregnated with silver nitrate (Figure 2).
Numerous microthrombi are observed in the lumens of the hemocapillaries; in this regard, a significant part of the hemocapillaries is characterized by blockage of the lumens. As a result of this process most of the hemocapillaries are switched off from the microcirculatory process and practically cannot participate in blood supply and gas exchange in the pancreas (Figure 2).
In some areas, along with destroyed and half-destroyed cellular elements, foci of lymphoid infiltration are registered, which are represented mainly by segmented leycocytes and lymphocytes. Hemocapillaries in the zones of destruction have free lumens,the endothelial lining is edematous, but the integrity of the cells is not impaired, the subendothelial membrane of the hemocapillaries has clear tinctorial properties.
It is necessary to indicate the narrowing of the lumens of arterioles and small arteries, hyperchromicity their middle membrane, swelling of the endothelial lining (Figure 2). 12 hours after modeling pancreatitis, the hemocapillaries become more winding, their lumens significantly increase due to filling with tissue fluid, containing the residues of tissue detritus (Figure 2). Along with lymphocapillaries, the lumens of which are free for lymphatic circulation, small vessels are registered, that contain small clots of lymph or coagulated tissue detritus in their lumens.
It is necessary to indicate the narrowing of the lumens of arterioles and small arteries, hyperchromicity their middle membrane, swelling of the endothelial lining (Figure 2). 12 hours after modeling pancreatitis, the hemocapillaries become more winding, their lumens significantly increase due to filling with tissue fluid, containing the residues of tissue detritus (Figure 2). Along with lymphocapillaries, the lumens of which are free for lymphatic circulation, small vessels are registered, that contain small clots of lymph or coagulated tissue detritus in their lumens.
Changes in larger lymphatic vessels are less expressed: everywhere large lymphatic vessels contain indigocarmine. As a rule lymphatic collectors are not subject to optically detectable destruction. In their lumens, microthrombi from coagulated lymph or tissues detritus are found less frequently. The degree of anastomosis of lymphatic vessels, the density of their distribution on unit of tissue surface remains within the modulatory disorders. This is evidence of continuous lymphatic drainage of the pancreas (Figure 2).
In subsequent periods of observation (1-3 days), cellular and interstitial edema is replaced by destruction of pancreatic components. 24 hours after modeling AP, structural changes become more distinct and are detected morphologically, as well as histochemically. In small foci of necrotic lesions, destructed and half-destructed mesotheliocytes, fragments of fibers and homogeneous complexes of tissue detritus are observed (Figure 3).
Morphological analysis of the blood stream of the pancreas showed that during the analyzed periods of experiments, the destruction of part of the mesotheliocytis causes blockage and exclusion of part of the hemocapillaries from the microcirculation. The lumens of the hemocapillaries are usually filled with the residues of tissue detritus or microthrombi from coagulated blood (Figure 3). One more circumstance attracts attention. As a rule, almost all hemocapillaries obstructed by microthrombi arise from foci of necrobiosis and necrosis of the pancreas. There are almost no capillaries filled with microthrombi or residues of tissue detritus in those areas, in which the destructive process is slightly expressed (Figure 3).
Larger blood vessels have a comparatively expressed resistance of the vascular wall. The main manifestation of hemodynamic disturbances in the pancreas is venous stagnation (Figure 3).
During the morphological analysis of the capillary wall of the lymphatic vessels, expressed cellular edema on the part of the endothelial lining of these vessels attracts attention. The majority of lymphatic vessels maintain their integrity and the process of lymphodynamics in them does not stop. In turn of, this creates optimal conditions for lymphatic drainage of the pancreas, which has been subjected to the inflammatory process. Obviously, inclusions of tissue detritus and fragments of halfdestructed cells are transported from indicated tissue as a part of the outflowing lymph. Despite the above mentioned changes, in this series the larger lymphatic vessels and lymphatic collectors almost do not undergo significant morphological changes; the vascular wall has the usual structural organization (Figure 3). Lymphcapillaries, the wall of which has been destroyed or is in the stage of irreversible damage, are registered in a single case. After 24 hours, the majority of lymphcapillaries are filled with indigocarmine solution, which indicates the preservation of their drainage function. The collecting lymphatic vessels and large main lymphatic trunks almost do not undergo morphological changes, because larger lymphatic vessels have the greatest tolerance to the destructive inflammatory process in the pancreas. Their wall preserves its typical structure, the sequence of distribution of structural elements, etc. Some edema of the endothelial lining of the lymphatic vessels, hypertrophy and increased argyrophility of the membrane are observed (Figure 3). lesion of the structural integrity of lymphcapillaries is registered relatively rarely and, as a rule, in areas of destruction of the structural components of the pancreas. Most lymphcapillaries preserve their passability. They are free from microthrombi or tissue detritus; indigocarmine complexes are constantly found in their lumens. This indicates that lymphatic drainage into the pancreas 24 hours after creating a model of AP functioning even in foci of necrosis and necro-biosis (Figure 3). 48 hours after modeling AP, morphological disorders, as a rule, extend to hemodynamic and lymphatic beds (Figure 4).
Regarding the microcirculation system, first of all, there is destruction and desolation of a significant number of hemocapillaries, and secondly, blockage of the lumen of these vessels with the residues of tissue detritus. In almost all capillaries, swelling and loss of tinctorial properties of the endothelial cells of the hemocapillars are detected. In those areas, where venous collectors were found, the presence of tissue detritus in the lumen of large vessels was revealed. As a result of desolation or destruction of hemocapillaries separate avascular zones of the pancreas are formed. As s rule, the processes of necrobiosis and necrosis actively unfold in these zones. Larger blood vessels also undergoes certain destructive changes. There is destruction of their endothelial lining, blockage of the lumen of these vessels with the residues of tissue detritus (Figure 4). In the zones of destruction there are many destroyed and halfdestroyed hemocapillaries, the presence of blood cells in the surrounding connective tissue, destruction of endothelial cells and subendothelial lining are noted. The collecting veins and small arteries have an edematous endothelial lining. In the perivascular zones these vessels have foci of lymphoid infiltration. Moreover, a significant number of small arterial and venous vessels are detected in the pancreas; there are microthrombi in their lumens (Figure 4). The lumen of majority of lymphcapillaries is free; sediments of indigocarmine are detected almost everywhere on the surface of the endothelium. In areas of necrosis, damaged lymhcapillaries are found; indigocarmine is not detected in most of them. The preserved part of these capillaries is mostly filled with the residues of tissue detritus, or the lumens are obstructed by microthrombi from coagulated lymph (Figure 4).
72 hours after modeling AP, cellular edema, swelling of fibrous connective tissue, foci of necrobiosis are registered in the pancreas. Destroyed and half-destroyed mesotheliocytes, the phenomena of necrobiosis and necrosis from the connective tissue are observed (Figure 5)
Destruction of the endothelium and basial membranes of hemocapillaries is accompanied by the destruction of mesotheliocytes and adjoining to them fibrous connective tissue (Figure 5). In areas where the processes of necrobiosis and necrosis are actively taking place, arterioles and venules are damaged. Larger arterioles, as a rule, preserve their structural integrity and do not have any special morphological abnormalities, even in areas of necrosis and necrobiosis (Figure 5).
In addition to the above mentioned two elements of the pathogenetic mechanism of pancreatitis, a third is added – the destruction of the lymhocapillaries, most of which are excluded from microcirculatory dynamics. Despite the fact that the structural integrity of large lymphatic vessels draining these collectors is preserved, the outllow of lymph is hampered due to violation of the integrity of small lymphatic vessels. The system of collecting lymphatic vessels is involved in the process of destruction; some of them have a damaged wall. In another part of these vessels, clots of tissue detritus or relatively large microthrombi are found, that turn off lymphatic drainage of various microregions of the pancreas (Figure 5).
Larger lymphatic vessels are better preserved; in most of them edema of the endothelial cell layer and swelling of the subendothelial base are registered. Almost all lymphatic vessels contain an indigocarmine mixture, which indicates their passability. The lumens of the lymphcapillaries are narrowed, but partial inflow of indigocarmine from the blood continues. There are many lymphcapillaries in the pancreas containing microclots of lymph that obturate their lumens, and turn them off from microcirculation. In some areas, the wall of the lymphatic capillaries is histologically damaged, the character of merger of the lymphcapillaries, their tinctorial properties are disrupted (Figure 5). lymphcapillaries that drain microregions with destroyed and half-destroyed cells and non-cellular elements are obturated. Some of the lymphcapillaries, originating from areas of the pancreas and preserving their structural integrity, passable for lymph and, as a rule, not obturated with microthrombi (Figure 5). It should be noted that in those zones where necrosis and necrobiosis of the morphological components of pancreas tissue are observed, the whole damaged zone is accumulations of tissue detritus (Figure 5).
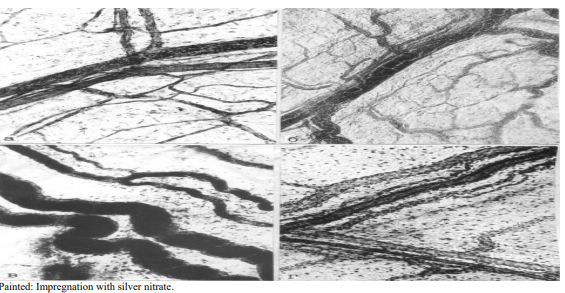
Figure 2: State of microhemolymphcirculation 12 hours after modeling acute pancreatitis: A) System of lymphatic and blood capillaries, occlusion of individual vessels by microthrombi; B) Hemocapillaries form a collecting venule, filled with formed elements of blood; C) A mass of indigocarmine fills large lymphatic vessels; D) The vascular net maintains the conditions for microcirculation in the lymph and hemocapillary system
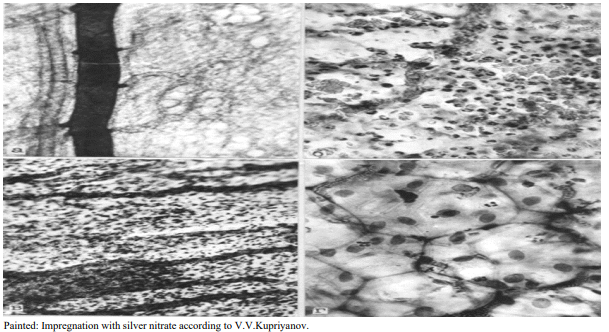
Figure 3: Microvasculatory bed of the pancreas 24 hours after modeling acute pancreatitis. A) Contrasting masses of indigocarmine fill the collecting lymhatic vessels: B) Zone of necrosis and necrobiosis, destruction of hemocapillaries and hemorrhage into crumbling pancreas; C) Microthrombosis of the collecting vein, occlusion of lymphcapillaries with tissue detritus; D) The intercellular spaces of most mesotheliocytes are impregnated with silver nitrate

Figure 4: Microvasculatory bed of the pancreas 48 hours after modeling acute pancreatitis. A) Some of the lymphcapillaries are filled with indigocarmine, other microvessels are free from contrast agent. B) A focus of destruction, which in addition to destroyed and half-destroyed cells contains numerous formed elements from the lumen of the hemocapillaries, the wall of which is lysed and destroyed; C) Indigocarmine fills the collecting lymphatic vessel; D) Only part of the blood and lymphatic capillaries preserves their micrometric parameters
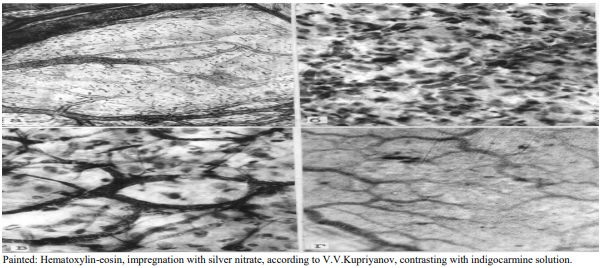
Figure 5: Structural organization of the pancreas microvasculatory bed 72 hours after modeling acute pancreatitis. A) Lymphatic collector and system of draining lymphatic vessels. Part of lymphcapillaries subjected to destruction; B) Total destruction of blood capillaries and hemorrhage into the area of the necrotic focus in the pancreas; C) Venous collector and destruction of part of the hemocapillaries with subsequent release of formed elements into the surrounding tissue; D) Indigocarmine fills only part of the lymphatic capillaries; empty and obstructed lymphatic capillaries are visible.
Findings
Thus, the results of experimental studies showed, that modeling of pancreatitis leads to pronounced structural and functional disorders in the microvasculatory bed of the pancreas. Already 12 hours after modeling, cellular edema is observed with a sharp narrowing of the interstitial and intercellular space and accumulation of fluid in the connective tissue. This leads to compression of the hemocapillaries, slowing of blood flow in the venules and, slightly in arterioles, the appearance of granularity in their lumen, and the opening of arteriole-venular shunts. Capillary stasis, aggregation of red blood cell occur with the formation of sludges and thrombosis of individual capillaries and venules with subsequent development of a block in the microcirculation system and the processes of exudation and transudation of the liquid part of the blood in the pancreas area within a day after modeling AP.
Simultaneously, as a result of stagnation, against the background of a developing block in the microhemocirculation system, the lymphcapillaries become more tortuous in their lumens, numerous microthrombi from the residues of tissue fluid and tissue detritus are registered. The lumen of lymphcapillaries expands due to the structural features of their walls, namely: a) by the presence of fiber threads; with their help, they are connected to interstitial tissue, which is during edema contributes to their expansion; b) by the “rounding” of endothelial cells with widening of intercellular spaces and; c) by the absence of a basial membrane, which facilitates the passage of fluid and microparticles into the lumen of the lymphcapillaries.
The drainage function of the microlymphcirculatory (MLC)-link of the MC system is activated.
In subsequent periods (2-3 days) cellular and interstitial edema is replaced by phenomena of destruction. The phenomena of necrosis and necrobiosis of connective tissue are accompanied by blockage, desolation and destruction of hemo-and of part of the lymphcapillaries, which leads to the formation of a vascular zones and is the reason for the exclusion of entire microregions of the pancreas from drainage. Hemorrhage and plasmatic permeation of the interstitium are detected throughout the entire field of view. On the third day, the microhemocirculation (MHC)-link of the MC system loses its structure: only arteriovenular shunts continue to function, while the MLC-link mainly continues to carry out its drainage and transport function.
Conclusions
During the first day after modeling AP, against the background of a developing blockage of the MHC-link of MC system, simultaneously activates the drainage and transport functions of the MLC-link of the MC system and the lymphatic system generally.
On the third day a complete blockage of the MHC-link of the MC system occurs, while the MLC-link, despite the destruction of part of the lymphcapillaries, mostly, continues to carry out its drainage and transport function.
The blockage of the MHC-link of the MC system with the simultaneous activation of the drainage and transport function of the MLC-link of MC system and the lymphatic system generally, identified at the morphological level, explains the low clinical effectiveness of conventional infusion-transfusional therapy for patients with AP, carried out by traditional methods (i/v, i/a, i/m), and this is the rationale for the use in this, an extremely severe category of patients with surgical profile endolymphatic therapy for the delivery of drugs to the site of inflammation and destruction of the pancreas, which consists in introducing drugs directly into the lymphatic system through a catheterized lymph vessel on the lower limb.
References
1.Beger, H.G. (2007). Severe acute pancreatitis: clinical course and management. World J Gastroenterol, (13), 5043-5051.
2. Daniel, M. Spagnolo., Phil, J. Greer., Celeste, Shelton Ohlsen., Shannon, Mance., Mitchell, Ellison, et al. (2022). Acute and Chronic Pancreatitis Disease Prevalence, Classification, and Comorbidities: A Cohort Study of the UK BioBank. Clinical and Translational Gastroenterology, 13, e00455.
3. Greenberg, J.A., Hsu, J., Bawazeer, M. et al. (2016). Clinical practice guideline: management of acute pancreatitis. Can J Surg 59(2), 128-140.
4. Gungabissoon, U., Delgado, M., Cooper, S., Ma Liyuan. Uings, I. (2021). The incidence of acute pancreatitis in the United States: identification of cases in an electronic healthcare database with supportive laboratory evidence. Pancreas. Journal of neuroendocrine tumors and pancreatic diseases and sciences. 2021; 50(8): e70–e72.
5. Johnson, C.D. (2015). Organ Failure and Acute Pancreatitis. In: Forsmark C., Gardner T., eds. Prediction and Management of Severe Acute Pancreatitis. New York: Springer; 2015.
6. Mandalia, A., Wamsteker, E., DiMagno, M. (2018) Recent advances in understanding and managing acute pancreatitis. F1000Res, 2018, 959.
7. Mantke, R., Lippert, H., Büchler, M.W., Sarr, M.G. (2013). International Practices in Pancreatic Surgery. Heidelberg: Springer, 2013, 206.
8.Papachristou, G., Muddana, V., Yadav, D., O’Connell, M., Sanders, M., et al. (2010). Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 105(2), 435-441.
9. Siebert, M., Fouler, A. Le., Sitbon, N., Cohen, J., Abba, J., Poupardin, E. (2021). Management of abdominal compartment syndrome in acute pancreatitis. Journal of Visceral Surgery, 158(5), 411-419.
10. Tomkötter, L., Erbes, J., Trepte, C., Hinsch, A., Dupree, A., et al. (2016). The Effects of Pancreatic Microcirculatory Disturbances on Histopathologic Tissue Damage and the Outcome in Severe Acute Pancreatitis. Pancreas, 45(2), 248-253.
11.. Yuping, R., Jun, R., Wei, S., Renshen, X., Yuhang, Ge., et al. (2021). Resveratrol Suppresses Severe Acute PancreatitisInduced Microcirculation Disturbance through Targeting SIRT1-FOXO1 Axis. Oxidative Medicine and Cellular Longevity, 2021, 8891544.
12. Wang, X., Liu, M., Hu, W., Cui, T., Yu, X., et al. (2020) Angiotensin-(1-7) Treatment Restores Pancreatic Microcirculation Profiles: A New Story in Acute Pancreatitis. Pancreas, 49(7), 960-966.



