Case Report - (2023) Volume 8, Issue 8
Giant Sacroccogieal Mature Teratoma Type 2, 40 Days Old Baby: Delayed Surgery Due to the Treatment of Newborn Injuries Which Occurred During Delivery-Case Report
2Clinic for Anesthesiology , Clinic Center of University Sarajevo, Bosnia and Herzegovina
3Department of Neonatal, Pediatric Clinic, Clinic Center of University Sarajevo, Bosnia and Herzegovina
4Clinic for Orthopedics and Traumatology, Clinic Center of University Sarajevo, Bosnia and Herzegovina
5Institute for Radiology, Clinic Center of University Sarajevo, Bosnia and Herzegovina
6Institute for Pathology, Clinic Center of University Sarajevo, Bosnia and Herzegovina
Received Date: Aug 14, 2023 / Accepted Date: Aug 23, 2023 / Published Date: Aug 31, 2023
Copyright: ©2023 K. Karavdic, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Citation: Karavdic, K., Begic-Kapetanovic, S., Milisic, E., Becic, E., Maksic, H., et al. (2023). Giant Sacroccogieal Mature Terato-ma Type 2, 40 Days Old Baby: Delayed Surgery Due to the Treatment of Newborn Injuries Which Occurred During Delivery-Case Report. J Clin Rev Case Rep, 8(8), 157-163.
Abstract
Introduction: Sacrococcygeal teratoma is an extragonadal germ cell tumor that develops in the fetal and neonatal periods and comprised of mixed elements derived from the three germ cell layers. A pathology-based classification is as benign (mature): much more common, comprising ~60-70%. Type II is extra-fetal with intrapelvic presacral extension a location-based on classification system according to the American Academy of Pediatric Surgery Section Survey.
Case report: A 2.25 kg cesarean delivered female, term neonate presented with large nonpulsatile, globular erythematous mass with lobulated surface and variable consistency. Magnetic resonance imaging of the lumbosacral spine showed multilocular cystic and solid lesion with foci of calcification and subtle communication with spinal canal. Histopathological examination showed mature endodermal, ectodermal, and mesenchymal elements such as cartilage, glial tissues, keratin cyst, and glandular elements with focus of primaryneuroepithelial and pancreatic elements. Entire tumor was excised. The baby expired secondary to wound infection.
Keywords
Teratoma, Sacrococcygeal, Mature, Type 2
Introduction
Sacrococcygeal teratoma is an extragonadal germ cell tumor that develops in fetal and neonatal age and is comprised of mixed elements formed from 3 germ cell layers. SCT develops from the sacrum and coccyx, protruding outwards and growing into the space of the pelvis. It is a very rare disease with a published incidence of 1 in 20000-40000 live births . Girls are much more represented, with a 3:1 to 4:1 female-to-male ratio [1].
Classification based on the pathohistological picture of the sacrococcygeal tumor divides it into the following: benign (mature): much more common, comprising 60-70% and malignant (immature). Based on the American Academy of Pediatrics Surgical.
Section survey (1962-1972), Altman et al. proposed in 1973. classification which included four types based on external component and intrapelvic/intra-abdominal extension of the tumor. Sacrococcygeal teratoma seen at birth are usually Altman Type I and II and rarely Type III [2].
The classification based on the location of the sacrococcygeal tumor presented by the American Academy of Pediatric Surgery Section Survey is: type I: developing only outside the fetus (can have small pre-sacral component); accounts for the majority of cases, 47% 12, type II: extra-fetal with intrapelvic presacral extension, type III: extra-fetal with extension through the pelvis into the abdomen and type IV: tumor developing entirely in the fetal pelvis [3].
Case Report
The female newborn was the third child from the third controlled pregnancy, the mother was 34 years old. Labor was at 40 NG, delivery was naturally, stimulated with Syntocin. After birth, the newborn’s weight was 5020 g, length 55 cm, Apgar 7/9. After delivery, the newborn was cyanotic, and oxygen support was administered. During the examination, the presence of a tumorous mass in the sacrococcygeal region, paresis of the left brachialis as well as suspected fractures of the left clavicle and left femur were verified. The female newborn was born in a regional hospital where she was immediately transferred to the Neonatology Department of the Pediatric Clinic. During further examination at the Neonatology, a cephalhematoma was verified on the right. Palpable crepitations in the area of the left clavicle. The left arm lags behind during adduction movements with external rotation of the hand. The left lower leg is larger in circumference with palpable crepitations.
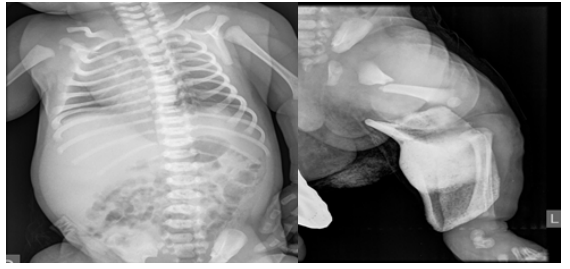
Figure 1A,1B: Fracture of the left clavicle and left femur.
In the sacrococcygeal region, a soft tissue tumor mass measuring about 10 cm in diameter is verified.
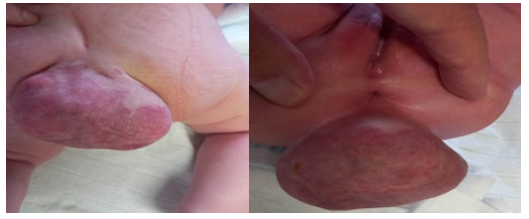
Figure 2A,2B: Sacrococcygeal a soft tissue tumor mass measuring about 10 cm in diameter.
A pediatric orthopedist is consulted, who places immobilization on the left lower extremity with the hip and knee flexed at 90 degrees. Immobilization for the left upper arm is also placed. After 5 days of immobilization and a control X-ray, Pavlik’s straps are placed and thus the conservative treatment of the fracture of the left femur continues. Pavlik belts are kept for 14 days, after which a control physical examination is performed, where swellings in the area of the left clavicle and left femur are palpated, which, when compared with the control X-ray, correspond to abundant callus formations. After 14 days of immobilization with Pavlik’s straps, physical exercises for stretching the shoulder joint and neck movement, as well as stretching the left hip and knee, began.
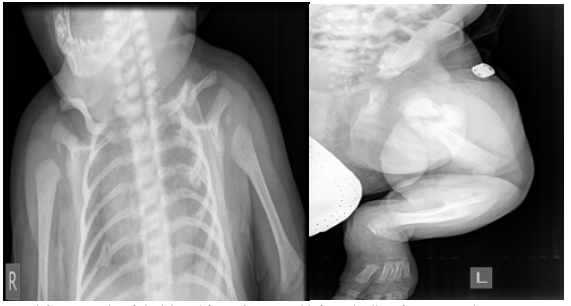
Figure 3A,3B: Satisfactory results of clavicle and femur fractures with formed callus after conservative treatment.
A pediatric cardiologist verifies moderately large ASD type sec. without volume loading of the right heart cavities.
After the completion of the orthopedic treatment and during the physical treatment, an Echo and MRI diagnosis of the pelvis is performed: “... in the area of the small pelvis presacral and precoccygeal, the existence of a zone of altered signal intensity is verified, which is mixed... and will correspond to cystic (hyperintense) and solid ( hypointense) zones....The total longitudinal diameter of the tumor is 106.6 mm. In the intrapelvic part, the laterolateral diameter is 36.4 mm, and in the extracorporeal part, the laterolateral diameter is 70 mm, while the largest AP diameter is 30 mm. In the pelvic area, the tumor is in contact with the back wall uterus, and the back and right wall of the rectum. The tumor mass has its own capsule.
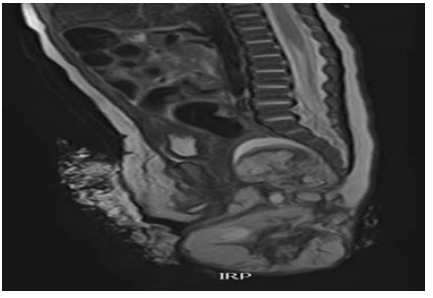
Figure 4: MRI shows tumor type II: extra-fetal with intrapelvic presacral extension.
The operative procedure is prolonged due to conservative treatment of the fracture of the left scapula and left femur. After the treatment is completed, surgery is indicated on the 40th day of life.
A Cehrvon incision is made over the sacrum and circularly 1 cm from the edge of the tumor and 2 cm from the edge of the anus. The tumor is prepared partly sharply and partly bluntly from the surrounding muscles and from the back of the front wall rectum.
The tumor originates from the inner side of the coccyx, which is resected and tied underneath artery and vein sacralis medie. The tumor is saddle-shaped and one part extends to the pelvis while the other part goes towards the left glute. The tumor is completely removed. Do lavage. A drain is placed rectal fascia, m.sphincter ani.ext and m.levator ani are reconstructed. The skin is reconstructed.
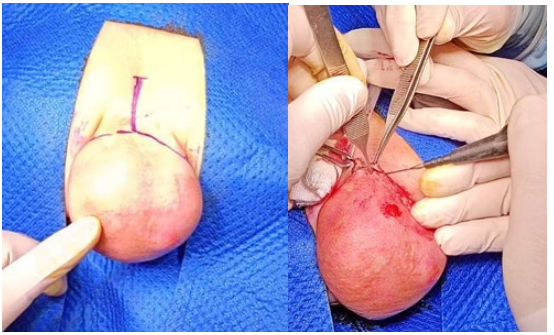
Figure 5A,5B: A Cehrvon incision is made over the sacrum and circularly 1 cm from the edge of the tumor and 2 cm from the edge of the anus
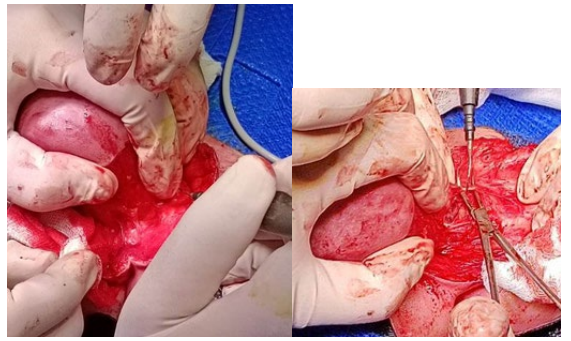
Figure 6: Prepareing partly sharply and partly bluntly from the surrounding muscles.
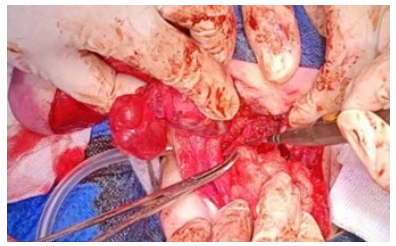
Figure 7: Resection of the coccygeal bone
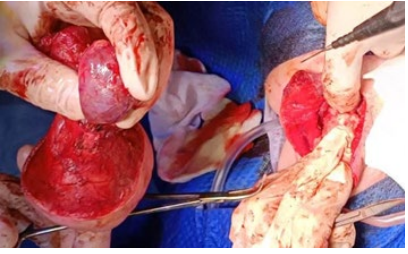
Figure 8: Tumor resection in toto.

Figure 9: Satisfactory cosmetic appearance of the gluteal region.
The obtained material was referred with the clinical diagnosis of “teratoma sacro coccigealis” according to Altman type II.A multinodular tissue sample with dimensions 105x75x60 mm, one part covered with skin measuring 65x60 mm, was obtained for analysis.on cross-section, the tissue is grayish-whitish, partly solid, partly cystic.Samples taken in the first download in 8 blocks, subsequently taken another 6 blocks, a total of 14 paraffin blocks.
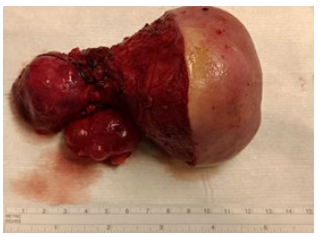
Figure 10: Removed tumor with intra- and extra-abdominal part.
Microscopically, the histological picture of the extragonadal teratoma, which is made up of three layers of germinal tissue, is present in the examined sections cells: ectoderm, mesoderm and endoderm; immature tissue components such as primitive neuroectodermal rosettes do not we find, and after downloading more cuts in 6 paraffin blocks. Fat, connective and muscle tissue, cartilage, bone, different types of epithelium, part of the cavities lining the cylindrical respiratory, cylindrical intestinal, squamous epithelium; from structures of cystically dilated follicles, nerve tissue.The resection margins marked with green ink include connective muscle and fatty tissue without a cystic component in the retrieved incisions of the surgical edges. Our findings align with the diagnosis of extragonadal teratoma.
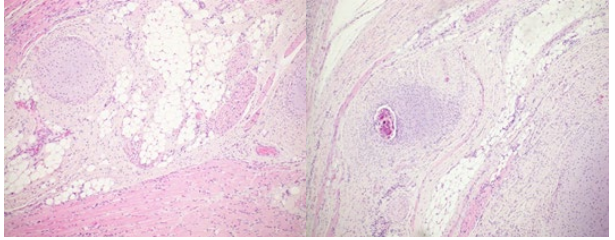
Figure 11: The microscopic evaluation demonstrates a plethora of tissues: fatty, bone, cartilage, fibrous, muscular, cylindrical respiratory and intestinal tissue; additionally, there are focuses with cystic dilated follicles and nerve tissue.
Postoperatively, the patient was placed in the Neonatal Intensive Care Unit. Immediately upon arrival from the operating room, the patient is extubated, dual antibiotic therapy and erythrocyte transfusion are prescribed. Oral nutrition is started early. On the third postoperative day, the subcutaneous drain is removed. On the 6th postoperative day, the patient was transferred from the Neonatal Intensive Care Unit to the Department of the Clinic for Pediatric Surgery. The wound heals partly per primam intentionem and partly per secundam intentionem. Sutures are removed on the 15th postoperative day. Control echo of the abdomen is normal. After 6 months of follow-up, AFP=101, Abdominal Echo normal. The pediatric hemato-oncologist recommended control AFP and Echo of the abdomen again in 6 months.
Discussion
The newborn with sacrococcygeal teratoma (SCT) has an excellent prognosis depending on the timing of diagnosis, malignant potential of the tumour and the ease of surgical resection. We had to delay the operation on the patient, only on the 40th day of age, due to the necessity of conservative treatment of fractures of the left clavicle and femur [1].
Antenatal diagnosis of SCT Altman Type I and II and even large Type III and IV are usually done by prenatal sonogram in the 24th-34th weeks of gestation Unfortunately, we had information that the tumor change was not diagnosed antenatally [2]. Diferent studies report a female to male ratio of 3-4:1, [3]. The case presented here is a female neonate.
Cesarean delivery is advised to a mother whose fetuses harbor large SCTs (>10 cm diameter) with high vascularity and in tumors larger than 5 cm in order to prevent the risk of rupture and bleeding [4]. Gynecologists at the regional hospital delivered naturally, stimulated with Syntocin. Although the tumor was detected antenatally as larger than 10 cm, the birth in the regional hospital was performed naturally, which led to the possibility of rupture and bleeding of the tumor. Although the tumor did not bleed or rupture during the natural birth, the fracture of the left clavicle and femur occurred, which led to the postponement of the primary treatment of the tumor.Altman et al. defined the size as small (2-5 cm), moderate (5-10 cm), and large (>10 cm) diameter and also classified on the basis of the extent of tumor. Size of hypervascular lesion often leads to congestive heart failure, coagulopathy from intramural bleeding, and consumption of clotting factors. The incidence of other congenital malformations associated with SCTs ranges from 5% to 26%, and the most common are anorectal and genital, but spinal dysraphism, sacral agenesis, and cardiac anomalies have been rarely noted [5]. Our case had Altman Type II large tumor mass without other congenital anomalies.
The recurrence rate without removal of the coccyx is as high as 37% [6]. Complete surgical excision of the tumor with coccygectomy is of paramount importance in the prevention of recurrence .During the operative procedure, we performed a complete resection of the coccygeal bone.During the operative procedure together with the resection of the intrapelvic and extracorporeal part of the tumor, we also resected its origin, the coccygeal bone.
Other complications include neuropathic bladder, bowel incontinence, constipation, and diarrhea. Septicemia due to wound infection is the most common cause of death in the early postoperative period [7]. The complication in our female newborn was partial wound dehiscence and its healing per secundam.
Poor cosmetic results in the buttock region are the most common longterm complication and can lead to distortion of body image, particularly in teenage, and can even cause psychological disturbances and depression. Providing good cosmesis may be challenging in the neonatal period due to lack of local tissue, large tumor, and thinned/stretched out muscles [8]. Our surgical team is extremely satisfied with the cosmetic result of the surgery.
The incidence of malignancy is more in large size SCTs,and Altman Type III/IV due to delay in diagnosis and presence of solid areas. Excellent survival rate has been reported in the literature; however, mortality rate for the mass larger than 10 cm is 18%. (9) Close follow up every 6 months, with physical examination including rectal examination, serum alphafetoprotein, and diagnostic imaging, is advisable for at least 3 years as chances of recurrence range from 11% to 22% even in completely excised mature neonatal SCT [10].
Conclusion
If a large tumor mass has been verified antenatally, delivery should be by cesarean section.
Natural delivery in patients with antenatally verified large tumor mass should be avoided due to possible tumor rupture, bleeding and injury to the musculoskeletal system.
If there are associated birth injuries, sacrococcygeal teratoma should be operated on after repairing the
References
1. Barksdale, E.M. Jr., & Obokhare, I. (2009) Teratomas in infants and children. Curr Opin Pediatr, 21, 344-349.
2. Schropp, K.P., Lobe, T.E., Rao, B., Mutabagani, K., Kay, G.A., Gilchrist, B.F., & Boles Jr, E.T. (1992). Sacrococcygeal teratoma: the experience of four decades. Journal of pediatric surgery, 27(8), 1075-1079.
3. Altman, R.P., Randolph, J.G., & Lilly, J.R. (1974). Sacrococcygeal teratoma: American Academy of Pediatrics surgical section survey-1973. Journal of pediatric surgery, 9(3), 389-398.
4. Tuladhar, R., Patole, S.K., & Whitehall, J.S. (2000). Sacrococcygeal teratoma in the perinatal period. Postgraduate medical journal, 76(902), 754-759.
5. Kamal, A., Mahmoud, S., & Tarek, B. (2006). Sacrococcygeal teratoma: a neonatal surgical problem.
6. Sinha, S., Sarin, Y.K., & Deshpande, V.P. (2013). Neonatal sacrococcygeal teratoma: our experience with 10 cases. Journal of Neonatal Surgery, 2(1).
7. Tuladhar, R., Patole, S.K., & Whitehall, J.S. (2000). Sacrococcygeal teratoma in the perinatal period. Postgraduate medical journal, 76(902), 754-759.
8. Gonzalez-Crussi, F., Winkler, R.F., & Mirkin, D.L. (1978). Sacrococcygeal teratomas in infants and children: relationship of histology and prognosis in 40 cases. Archives of Pathology & Laboratory Medicine, 102(8), 420-425.
9. Bilik, R., Shandling, B., Pope, M., Thorner, P., Weitzman, S., & Ein, S.H. (1993). Malignant benign neonatal sacrococcygeal teratoma. Journal of pediatric surgery, 28(9), 1158-1160.
10. Rescorla, F.J., Sawin, R.S., Coran, A.G., Dillon, P.W., & Azizkhan, R.G. (1998). Long-term outcome for infants and children with sacrococcygeal teratoma: a report from the Childrens Cancer Group. Journal of pediatric surgery, 33(2), 171-176.



