Case Report - (2024) Volume 9, Issue 1
Crohns Disease Complicated By Rectal Amyloidosis: A Case Report
2Department of Rdiology, Faculty of Medicine of Tlemcen, University of Tlemcen Aboubakr Belkaid, Algeria
3Department of Pathology, Faculty of Medicine of Tlemcen, University of Tlemcen Aboubakr Belkaid, Algeria
Received Date: Dec 19, 2023 / Accepted Date: Dec 26, 2023 / Published Date: Jan 03, 2024
Abstract
Digestive amyloidosis is a disease linked to the deposition in different organs of the digestive system of amyloid substance. There are two forms, AL amyloidosis or primary amyloidosis; A for amyloidosis and L for immunoglobulin light chain and amyloidosis secondary to chronic inflammation is the AA phenotype. We report the case of a patient with no major medical history suffering from chronic colic Crohn’s disease, with a long history of medical treatments before the introduction of anti-TNFα.mis, who was complicated by rectal AA amyloidosis, this this incidental discovery on biopsy of the rectal submucosa, without another identifiable underlying etiology.
Keywords
Crohn's disease, Hypoalbuminemia, Proctoscopy, Congo red staining, Amyloidosis
Abbreviations
CD:Crohn’s disease; IBD: Inflammatory bowel disease; AA: Amyloid-associated amyloidosis; FOGD: eso-gastro_duodenal fibroscopy; SAA: Serum Amyloid A.
Introduction
Secondary digestive amyloidosis is a rare but serious complication of Crohn's disease, usually seen in inflammatory bowel disease (IBD). At least 1% of patients with Crohn's disease (CD) develop amyloidosis. In the literature, the time between the onset of Crohn's disease and the diagnosis of amyloidosis varies from one to 21 years. The symptoms that occur depend on the organ affected. The use of anti-TNFα may improve the prognosis of rectal AA amyloidosis.
Observation
A 48-year-old man, a chronic smoker with a history of drug allergy to ciprolon and cholecystectomy, followed in our department for CD diagnosed 8 years ago. In its inflammatory form of total colon site, treated with infliximab after failure of conventional treatments (5ASA, Corticotherapy, immunosuppressants), is hospitalized in our department for gastrointestinal disorders. Acute involving fecal fluid diarrhea without fever, vomiting of food, deterioration in general condition showing weight loss of 6 kg. A notion of convulsive seizures having caused a fall in height has been reported.
2.1 Admission Exam
Clinical examination: Conscious patient, uncooperative, presenting dysarthria, anicteric ideomotor slowness, stable hemodynamic constants, afebrile, BMI: 24, Glasgow score 15/15. Presence of psoriasis on the scalp, face, trunk, back (Figure 1). Examination of the oral cavity revealed the presence of three ulcers and absence of glossitis.
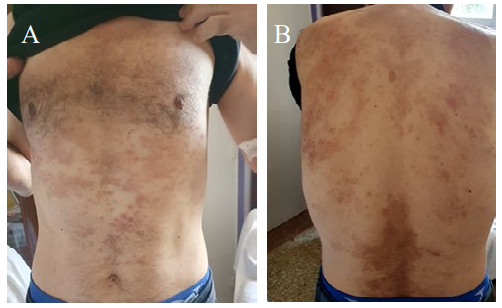
Figure 1: A) Psoriasis lesions in the thorax and abdomen image; B) Psoriasis lesions on the back
On palpation, the abdomen was supple, no hepatosplenomegaly, no abdominal mass. The proctological examination revealed the presence of an old painless perianal fissure (Figure 2).
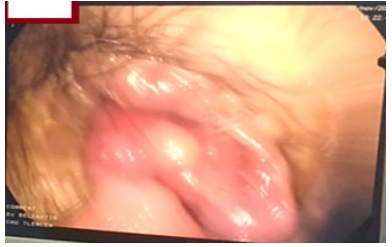
Figure 2: Perianal fissure at midday.
The rest of the physical examination did not find any palpable superficial lymphadenopathy. On the neurological level, a notion of paresthesias of the lower limbs like tingling, and an absence of walking or balancing problems. Osteo-tendinous reflexes were present in all 4 limbs, with no motor deficit. Faced with the presence of a non-painful red eye, an ophthalmological examination using a lamp was carried out revealing uveitis. On the osteoarticular level: unilateral left knee pain.
2.2 Diagnostic approach
The biological assessment carried out revealed (Table 1) normocytic normochromic anemia, thrombocytosis reactive to inflammation.
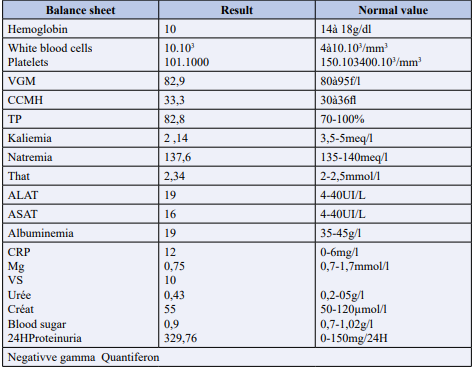
Table 1: Patient's biological data on admission.
A disturbed blood ionogram with hypokalemia, severe hypoalbuminemia at 19 gr/l, slightly positive CRP and moderate proteinuria, renal function was normal. The etiological investigation of this hypoalbuminemia including a cardiac assessment, 24-hour proteinuria was negative, and the causes of exudative enteropathy were eliminated, viral serologies B, C, HIV, CMV, HSV, EBV came back negative. Morphologically, one Frontal telethorax, and the standing abdomen without preparation were without particularities, apart from aerocolia. The brain and thoracic CT scan was without any particular organic cystic or infectious broncho-pulmonary, mediastinal and/or parietal anomalies.
The abdominal-pelvic scan revealed gastric wall thickening (Figure 3).
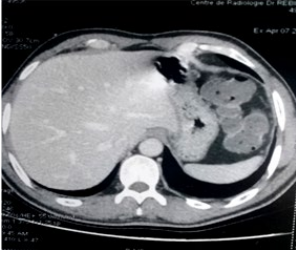
Figure 3: Abdominal CT in axial section, shows gastric thickening
We completed the etiological investigation of this gastric thickening revealed on abdominal CT, by carrying out an oesogastroduodenal fibroscopy, which showed a blistered congestive antro-fundal mucosa with some antral erosions, a regular post bulbar circumferential narrowing, passable as well as duodeno-gastric bilious reflux. Fundal, antral and post bulbar biopsies were carried out, the Histo-pathological study of which was normal in appearance, non-specific duodenitis with absence of Helicobacter pylori infestation.
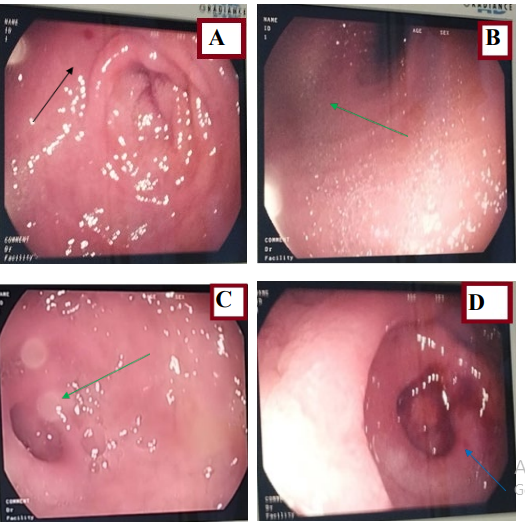
Figure 4: Esophagogastroduodenal fibroscopy. A,B) show a congestive, swollen antral mucosa with some erosions; C,D) show regular post bulbar narrowing.
To explore the diarrhea, an ilecolonoscopy was carried out which revealed multiple erosions disseminated throughout the entire colon, predominantly at the level of the rectum, with a blistered appearance, edema of the mucosa, and the presence of some superficial rectal ulcerations (Figure 5).
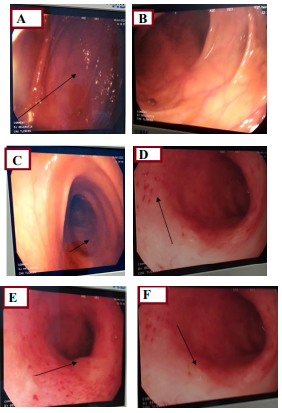
Figure 5: A,B,) Show a congestive colonic mucosa in places with an edematous appearance; D,E,F) Shows congestive rectal mucosa with multiple erosions.
Multiple staged biopsies taken, concluding in flare-up Crohn's disease and amyloidosis. The diagnosis of rectal AA amyloidosis was established by rectal biopsies (Figure 5) which found a rectal mucosa bordered by a simple columnar epithelium surmounting a chorion remodeled by a polymorphous interstitial lympho-plasmacytic inflammatory infiltrate mixed with turgid capillaries and a discreet peri-glandular and interstitial hyaline fibrosis. Congo red staining highlights a deposit of amorphous eosinophilic amyloid substance at the level of the basement membrane and around a few glands.
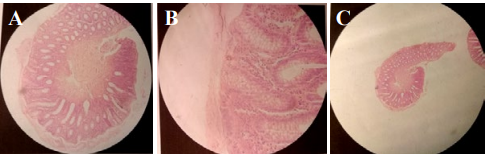
Figure 6: A,B,C) Rectal biopsy; H,E) Staining (Routine pathology lab staining).
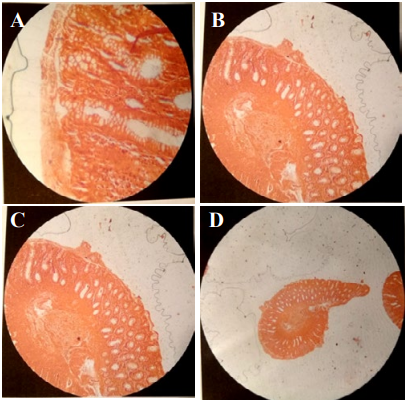
Figure 7: A,B,C,D) Rectal biopsy Congo red staining. Histological appearance suggests rectal amyloidosis
As part of the assessment of small bowel disease, an entero-CT scan was carried out which found thickening of the small bowel and colic loops, without fistulating or stenosing small bowel disease (Figure 8 and 9).
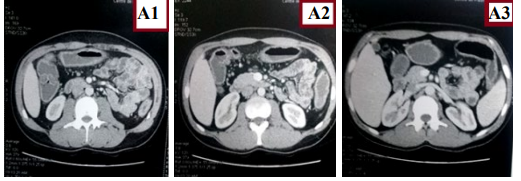
Figure 8: A1,A2,A3) Axial CT scan shows colonic thickening.
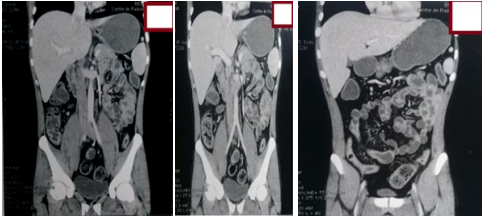
Figure 9: Frontal CT scan shows ileitis with diffuse colonic thickening.
Other examinations were carried out to look for disseminated amyloidosis, by an Echocardiography which returned normal as well as the dosage of Troponin I and T, and a thyroid ultrasound which returned normal.
2.3 Therapeutic intervention and follow-up
Upon admission, the patient was placed on parenteral nutrition for a few days, with correction of electrolyte disorders until normalization of serum potassium, albumin supplementation, and a therapeutic regimen established based on IV corticosteroids 100 mg HHC/8 hours associated with double antibiotic therapy flagyl 500mg/8 hours, claforan 1g/8 hours, and anticoagulation with lovenox 0.4UI/SC/12H. For the current flare-up of his CD, biotherapy based on infliximab 5mg/kg was administered coinciding with his treatment according to his initial plan.
The evolution under antibiotic therapy, corticosteroid therapy, anticoagulation and infliximab was progressively favorable towards the healing of the new outbreak of his CD, marked by a normalization of CRP, a correction of hypoalbuminemia and a remarkable improvement in his general condition with restoration of his language and motor activity.
Discussion
Rectal AA amyloidosis is defined by the deposition of an amyloid substance in the mucosa and submucosa of the rectum [1,2]. It is often underdiagnosed, and in the vast majority of cases goes unnoticed [2,3]. There is an association with IBD since protein A, responsible for the formation of amyloid deposits, is released during periods of disease activity causal inflammatory. Prolonged inflammation is a prerequisite for the development of AA amyloidosis, because the majority protein of the deposits is a fragment of the serun amyloid-associated protein (SAA), one of the most important proteins in the inflammatory reaction, but, other factors, mainly genetic, are involved in the susceptibility to the occurrence of AA amyloidosis [3].
During IBD, the prevalence of amyloidosis (AA) is rather rare and is around 0.5% [4], however in the case of CD, the incidence is much higher estimated at 1% [5,6]. In our patient, the diagnosis of MC had preceded that of AA. The median time between the diagnosis of CD and that of amyloidosis is extremely variable (from 1 to 37 years) [7]. In our case, it was made after eight years of progression of his CD. The risk factors identified in the literature are severe forms, male gender, fistulizing phenotype of CD, anoperineal lesions (APL) and damage extradigestive [4]. Our observation relates several of these factors, regarding a male case, who had presented a severe flare-up of his CD, associated with multiple extradigestive manifestations such as operated gallbladder lithiasis, extensive psoriasis, cutaneous manifestations such as recurrent oral ulcers, ocular damage represented by uveitis, and joint damage.
The presenting symptomatology is atypical and has several aspects associating chronic diarrhea [7,8], very common in AA forms, by inhibition of the autonomic nervous system, chronic bacterial colonization, malabsorption of bile acids with steatorrhea or even ischemia. Secondary to vascular infiltration [9,10]. Rectal digestive damage is not always responsible for symptoms, the clinical context is sometimes suggestive. It can be responsible for diarrhea, weight loss, lack of appetite and axonal neuropathy starting in the extremities [8].
In our patient, the clinical signs were dominated by fecal diarrhea with weight loss, acute vomiting, the origin of this diarrhea may be due to a microbial proliferation falling within the framework of his illness, by an exudative enteropathy or by malabsorption of bile salts because our patient is cholecystectomized. The etiological search for the causes of vomiting, by the practice of FOGD and gatro-duodenal biopsies did not reveal gastro-bulbar parietal involvement. In case of gastro-duodenal involvement, the appearance found is granular (70% in the duodenum) or polypoid, gastric erosions (57%), which were visualized in this clinical case as well as thickened folds [9-11]. However, the histo-pathological study is almost always positive.
In our case, small bowel disease on the entero-CT scan was related to the new outbreak of his disease, ileo-colonoscopy was indicated with staged biopsies at the different colonic and rectal segments, the latter confirmed AA rectal amyloidosis. The diagnosis of rectal amyloidosis is histological [3,11]. This rare pathology can be detected with precision on systematic biopsies of the rectal submucosa which have a sensitivity of 85 to 97% [3,11] as part of an extension or surveillance assessment of IBD. Biopsies are positive in more than 80% of cases [11,12].
Confirmation of the diagnosis in our patient was made by Congo red staining, showing visible HES deposits of the dye in the betapleated structure. Immunostaining helps classify amyloidoses into their different histological types, but they are not always carried out in current practice. In cases of colonic involvement, which is secondary to colonic parietal ischemia due to amyloid deposits in the muscularis mucosae and in the submucosa. The symptomatology is rather noisy with occlusive attacks, a picture of stenosis, volvulus, Ogilvie syndrome or perforation. Colonoscopy is the rule [11] objective several macroscopic aspects generally represented by a granular appearance of the mucosa (34%), colonic stenosis, loss of haustrations, mucosal thickening of the folds, even a nodular appearance, polyps, erosions (11%) or ulcerations (9%) [11,12].
In our patient, superficial erosions and ulcerations were found in the rectum, as well as erosions. According to international recommendations, the evaluation and search for damage to other organs of the associated digestive system [13,14] or other organs is systematic because it conditions the vital prognosis, is done by renal and hepatic function, proteinuria, echocardiography. Scintigraphy with the radiolabeled SAP component can quickly and specifically locate amyloid deposits allowing real mapping. Hepatic and splenic locations are most often visualized. SAP component scintigraphy can provide a diagnostic argument in favor of amyloidosis when histological studies are negative. Scintigraphy with the P component marked at 123 [13-15].
Therapeutically, the treatment of rectal amyloidosis is not well codified [16,17]. It is mainly based on etiological treatment, by fighting against the intestinal inflammation of the underlying pathology.
To powerful immunosuppressive drugs, with non-specific action, and major short-and medium-term adverse effects (mainly cyclophosphamide and chloraminophen) is no longer relevant as first-line treatment. Biotherapies are now the most widely used therapies [17,18]: essentially anti-TNF, anti-IL-1, anti-IL-6R. Due to the rarity of AA amyloidosis complicating IBD, there is a lack of clinical trials comparing the effectiveness of these treatments. Only case series have been reported. In our observation, the use of anti-TNF had made it possible to obtain a rapid and good clinical response, with the endpoint of the disappearance of clinical signs, the improvement of biological abnormalities in particular hypoalbuminemia and the correction of other disorders. The responses are most often evaluated in the short term or medium term, by measuring fecal calprotectin and CRP every three months.
Remission of a flare-up of CD disease associated with rectal AA amyloidosis is possible, this case illustrates it well, the benefit is all the more important as the treatment is early and allows the complete disappearance of the inflammatory syndrome [17]. The indication for colchicine deserves to be discussed, especially since the treatment of the causative disease does not allow, in the majority of cases, remission of amyloidosis [16,17,19,20].
The progression of amyloidosis is severe, linked to amyloid deposits in other organs, which are often irreversible [20]. The prognosis is guarded for AA amyloidosis, particularly in cases of cardiac involvement (with elevated troponin T and NT proBNP), renal [19]. In addition to other predictive factors of poor prognosis which are hyperbilirubinemia, thrombocytopenia, anemia, weight loss and high number of affected organs. There is no correlation between the extent of amyloid deposits and their toxicity. In fact our patient remained asymptomatic for more than a year. Subsequently, he presented an episode of hypoalbulinemia corrected under albuminotherapy.
Conclusion
Rectal amyloidosis secondary to CD is a rare disease that is often overlooked and difficult to diagnose. The diagnosis is histological. Targeted biopsies of the rectum with Congo red staining must be carried out. Treatment is based on controlling inflammation, particularly with antibiotics and biotherapies. The search for proteinuria and the measurement of plasma creatinine remain the key elements for screening for rectal amyloidosis during IBD.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Author Contributions
All authors approved the final version of the manuscript.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Declarations
Ethics approval and consent to participate this study was conducted in accordance with the Declaration of Helsinki and approved by the research and ethics committee of Algeria; reference number is not applicable. This study does not contain any personal information that could lead to the identification of the patient.
Consent for Publication
Written informed consent to publish this information was obtained from the patient of the study
Acknowledgement
None.
References
1. Kyle, R.A. (1982). Amyloidosis. Clin Haematol, 11, 151- 180.
2. Tosca Cuquerella, J., Bosca-Watts, M.M., Anton Ausejo, R., Tejedor Alonso, S., Mora De Miguel, F., Minguez Perez, M. (2016). Amyloidosis in Inflammatory Bowel Disease: A Systematic Review of Epidemiology, Clinical Features, and Treatment. J Crohns Colitis, 10, 1245-1253.
3. Senecal, J.B., Abou-Akl, R., Allevato, P., Mazzetti, I., Hamm, C., Parikh, R., Woldie, I. (2023). Amyloidosis: a case series and review of the literature. J Med Case Rep, 17, 184.
4. Kyle, R.A., Bayrd, E.D. (1975). Amyloidosis: review of 236 cases. Medicine (Baltimore), 54, 271-299.
5. Venturin, C., Chauvenet, M., Phelip, G., Belkhodja, H., Bouchra, A., Flourié, B., Nancey, S., Boschetti, G. (2018). Amylose digestive : du diagnostic au traitement. HépatoGastro & Oncologie Digestive, 25, 451-460.
6. Grateau, G. (2000). Amyloses : manifestations et lésions vasculaires. Sang Thrombose Vaisseaux, 12, 453-458.
7. Basturk, T., Ozagari, A., Ozturk, T., Kusaslan, R., Unsal, A. (2009). Crohn’s disease and secondary amyloidosis: early complication? A case report and review of the literature. J Ren Care, 35, 147-150.
8. Tada, S., Iida, M., Iwashita, A., Matsui, T., Fuchigami, T., Yamamoto, T., Yao, T., Fujishima, M. (1990). Endoscopic and biopsy findings of the upper digestive tract in patients with amyloidosis. Gastrointest Endosc, 36, 10-14.
9. Tada, S., Iida, M., Yao, T., Kawakubo, K., Yao, T., Fuchigami, T., Okada, M., Fujishima, M. (1994). Gastrointestinal amyloidosis: radiologic features by chemical types. Radiology, 190, 37-42.
10. Gertz, M.A. (2016). Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol, 91, 947-956.
11. Vanderschueren, S., Del Biondo, E., Ruttens, D., Van Boxelaer, I., Wauters, E., Knockaert, D.D.C. (2009). Inflammation of unknown origin versus fever of unknown origin: two of a kind. Eur J Intern Med, 20, 415-418.
12. Cabezuelo, J.B., Egea, J.P., Ramos, F., Torrella, E., Muray, S., Alcázar, C. (2012). Infliximab in the treatment of amyloidosis secondary to Crohn’s disease. Nefrologia, 32, 385-388.
13. Masson, E., n.d. Moyens paracliniques du diagnostic des amyloses [WWW Document]. EM-Consulte.
14. Lachmann, H.J., Goodman, H.J.B., Gilbertson, J.A., Gallimore, J.R., Sabin, C.A., Gillmore, J.D., Hawkins, P.N. (2007). Natural history and outcome in systemic AA amyloidosis. N Engl J Med, 356, 2361-2371.
15. Madsen, L.G., Gimsing, P., Schiødt, F.V. (2009) Primary (AL) amyloidosis with gastrointestinal involvement. Scand J Gastroenterol, 44, 708-711.
16. Halm, U., Berr, F., Tannapfel, A., Klöppel, R., Secknus, R., Mössner, J. (1998). Primary amyloidosis of the mesentery and the retroperitoneum presenting with lymphedema. Am J Gastroenterol, 93, 2299-2300.
17. Hayman, S.R., Lacy, M.Q., Kyle, R.A., Gertz, M.A. (2001). Primary systemic amyloidosis: a cause of malabsorption syndrome. Am J Med, 111, 535-540.
18. Gould, M., Zarrin-Khameh, N., Sellin, J. (2013). Small bowel amyloidosis. Curr Gastroenterol Rep, 15, 350.
19. Lowdell, C.P., Shousha, S., Parkins, R.A. (1986). The incidence of amyloidosis complicating inflammatory bowel disease. A prospective survey of 177 patients. Dis Colon Rectum, 29, 351-354.
20. Gilles Grateau, Jean-philippe bastard, Jean-jacques boffa, David buob, Soraya Fellahi, Alexandre Karras, et al. PNDS amylose AA Amyloidosis in podcast version (available on your various usual listening platforms).
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



