Original article - (2024) Volume 9, Issue 11
Clinical Impact of Deuterium Depletion on Survival Outcomes in Pancreatic Cancer
2Department of Public Health, Faculty of Medicine, University of Szeged, Szeged, Hungary
Received Date: Oct 29, 2024 / Accepted Date: Nov 07, 2024 / Published Date: Nov 20, 2024
Copyright: Copyright: ©2024 Beáta Zs. Kovács, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Citation: Kovacs, B.Zs., Papp, A., Somlyai, I., Somlyai, G. (2024). Clinical Impact of Deuterium Depletion on Survival Outcomes in Pancreatic Cancer. J Clin Rev Case Rep, 9(11), 01-07.
Abstract
Pancreatic cancer ranks as the 12th most common cancer globally, responsible for 7% of all cancer-related deaths. Patients with unresectable tumors have only a 20% chance of surviving one year; patients diagnosed at stage III or IV of the disease expect only 4 to 6 months to live. This retrospective study evaluated the impact of deuterium-depleted water (DDW) on the median survival time (MST) of 93 patients with pancreatic cancer. Without altering their conventional treatments, the patients’ daily fluid intake was entirely replaced with 1.5 to 2 liters of DDW to lower deuterium concentration in their bodies. The MST from diagnosis to the end of observation was 21.5 months (95% CI: 12.6–30.3). Notably, there was a pronounced, though not statistically significant (p = 0.215), difference in MST between genders. The MST for female patients was 37.5 months (95% CI: 8.8–66.1), compared to 17.1 months (95% CI: 12.1–22.1) for male patients. Four case studies are presented, highlighting significantly longer survival times than previously reported in the literature. These findings suggest that deuterium depletion, when combined with standard treatments, may improve survival outcomes in advanced-stage pancreatic cancer more effectively than targeted therapies or combination treatments alone.
Keywords
Deuterium, Deuterium depletion, Deuterium-depleted water (Ddw), Pancreatic cancer, Submolecular regulatory system (Smrs), Median survival time (Mst)
Introduction
Pancreatic cancer is an aggressive malignancy with a poor prognosis. Surgery remains the most effective treatment, but only 10-15% of patients are eligible because 80% are diagnosed when the disease is already unresectable [1]. Even in cases of successful complete resection, long-term survival is limited [2- 4]. For patients with unresectable tumors, the one-year survival rate is only 20%, even with single-agent chemotherapy [5]. Furthermore, 95% of patients diagnosed at stage III or IV face a life expectancy of merely 4 to 6 months [6].
A submolecular approach may offer a new avenue for cancer treatment. Studies have shown that reducing natural deuterium levels via deuterium-depleted water (DDW) induces apoptosis in vitro and causes tumor regression in vivo [7-9]. The role of deuterium in cell cycle regulation and its effect on protooncogenes and tumor suppressor genes (e.g., C-Myc, Ha-ras, p53) has been multiplely confirmed [10-13]. Cancer cells are highly sensitive to deuterium depletion, which can lead to tumor necrosis [14]. In prospective and retrospective studies DDW significantly increased median survival time, with some stage IV patients surviving 5-10 years after starting DDW [15-20].
However, pancreatic cancer exhibits a behavior that is markedly different from other cancer types studied and published to date, proving to be the most resistant to deuterium depletion. Nevertheless, the efficacy of DDW has also been demonstrated in pancreatic cancer. Boros et al. reported that deuterium depletion alone inhibits the growth of MIA PaCa-2 pancreatic cancer cells in vitro by regulating NADPH-dependent reductive synthesis through the oxidative pentose phosphate pathway [21]. In patients with advanced pancreatic cancer, a reduction in serum deuterium levels achieved by consumption of DDW, alongside conventional therapies, led to a threefold increase in median survival time (MST). Patients who received DDW treatment (n=56) had an MST of 19.6 months, compared to 6.36 months in those treated with chemotherapy alone (n=30). There was a strong and statistically significant Pearson correlation (r=0.504, p<0.001) between survival time and the duration of DDW treatment.
We present a retrospective follow-up study to assess the impact of DDW consumption on the outcomes of pancreatic cancer patients. In this study, the daily fluid intake of 93 patients with pancreatic cancer was replaced with DDW without any modification to their conventional treatment regimens. The primary endpoint of the study was MST. Various subgroups were analyzed to compare the results with historical controls.
Materials and Methods
Administration of Deuterium-depleted water
All patients consumed DDW in the form of commercially available food; Preventa deuterium-depleted drinking water with different deuterium concentrations. The production process and product properties have been previously described [16,22]. The treatment aimed to lower the patients’ deuterium levels by replacing their regular fluid intake with 1.5-2 liters of DDW daily. DDW was used as a supplement to conventional therapies, not as a replacement. Patients typically began with a DDW concentration of 85 ppm D, which was gradually reduced to 65, 45, and 25 ppm for 1 to 3 months to achieve a continuously decreasing serum deuterium level.
The duration of DDW consumption in the follow-up cohort ranged from 3 to 981 days, with a cumulative consumption time of 19,538 days (equivalent to 53.53 years). The total follow-up period, from diagnosis to the end of observation, was 46,882 days (128.1 years).
Data collection
Our retrospective study included data from 93 pancreatic cancer patients (51 females and 42 males) who consumed DDW between March 1995 and October 2022. The patients had an average age of 61 years (median: 62 years) and an average body weight of 67.8 kg (median: 66 kg). The evaluation was based on the following information: date of diagnosis, date of beginning and end of DDW consumption, date of the end of follow-up, and the patient’s status (alive or deceased) at the time of the last update.
All patients received conventional treatments and underwent follow-up examinations at various hospitals in Hungary. Data collection was finalized in October 2022. Patient records were reviewed retrospectively, with survival being the sole endpoint of the study.
Statistical evaluation
The Kaplan-Meier method was used for the general statistical analysis to estimate survival. The analysis was conducted using MedCalc Statistical Software, Version 12.3.0. Since this was a retrospective study with patients starting DDW consumption at different time points after their diagnosis, MST was calculated both from the time of pancreatic cancer diagnosis and the initiation of DDW consumption. MST was also analyzed separately for male and female patients.
The Pearson correlation method was used to analyze the relationships between DDW treatment, months of survival, and body weight. Adware Research Ltd. (Balatonfüred, Hungary) conducted the statistical evaluation.
Results
3.1. Retrospective study with all 93 pancreatic cancer patients and selected subgroups receiving conventional therapies and consuming DDW
The 93 patients evaluated in this study were all diagnosed with pancreatic cancer before beginning DDW consumption. At the start of DDW treatment, all patients had detectable tumors except for four who had previously undergone surgery. The cumulative follow-up time from diagnosis to the end of the study was 128.1 years. The total time between diagnosis and the initiation of DDW consumption was 49.9 years, with an average time gap of 6.4 months. The cumulative duration of DDW consumption was 53.5 years, with individual treatment periods ranging from 1 day to a maximum of 981 days. Patients began DDW consumption at varying intervals after diagnosis, from 0 to 2,331 days.
3.1.1. Calculations of MSTs in a total of 93 pancreatic cancer patients: The 93 patients consuming DDW in addition to conventional treatments showed increased MST compared to historical control (4-6 months) in the overall population, MST from the establishment of diagnosis to the end of observation was 21.5 months (95% CI: 12.6–30.3) [1,5].
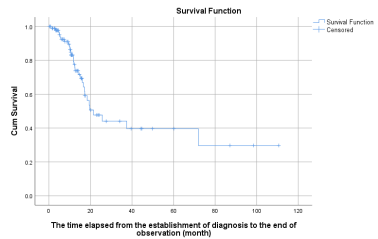
Figure 1: Kaplan-Meier graph of the survival of the 93 pancreatic cancer patients showing a 21.5 (95% CI: 12.6 – 30.3) months MST compared to the expected 4-6-month MST based on the latest statistics of pancreatic cancer worldwide. The hash marks on the survival curve indicate when a patient was censored
3.1.2. Median Survival Time (MST) in the entire cohort by gender: The median survival time (MST) analysis indicated a notable, though not statistically significant, difference between genders (p=0.215). The MST from diagnosis to the end of observation was 37.5 months (95% CI: 8.8–66.1) for females, compared to 17.1 months (95% CI: 12.1–22.1) for males. The study population was composed of 55% females and 45% males, with mortality rates of 31% among women and 33% among men during the follow-up period. Consequently, the twofold longer MST observed in the female subgroup cannot be attributed to differences in mortality rates.
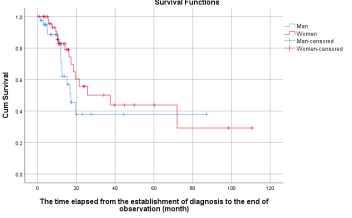
Figure 2: Kaplan-Meier graphs of survival in the female and male subgroups of the whole patient population. The MST from the establishment of diagnosis to the end of the observation was 37.5 months (95% CI: 8.8–66.1) in the female subgroup and 17.1 months (95% CI: 12.1–22.1) in the male group.
3.1.3. Correlation between survival times and DDW consumption in the entire cohort: A strong, statistically significant correlation was found between the duration of DDW treatment and the time from diagnosis to the end of observation (r=0.520, p<0.001; Figure 3a). Similarly, a significant correlation was observed between the length of DDW consumption and the time from the start of treatment to the end of observation (r=0.694; Figure 3b).
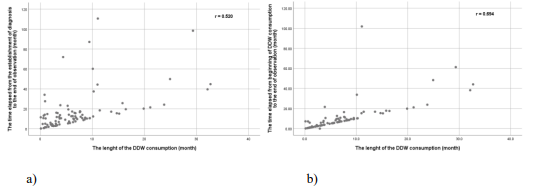
Figure 3: Correlation between observation time and duration of DDW consumption in the study population. In graph a) survival is defined as the time from diagnosis to the end of observation, showing a correlation of r = 0.520. In graph b) survival refers to the time from the start of DDW consumption to the end of observation, with a stronger correlation of r = 0.694
3.1.4. Correlation between body weight and DDW treatment in the whole cohort: Body weight strongly influences the rate of decrease in serum D concentration in DDW-consuming patients, so the correlation between body weight and the length of DDW consumption was investigated. In our study population, however, there was no statistically significant correlation between body weight and the length of the DDW consumption (r = -0.031, p = 0.769) (Figure 4)
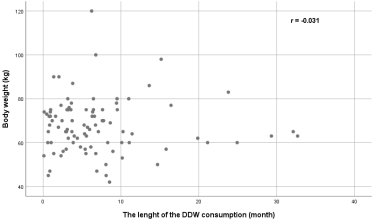
Figure 4: A lack of correlation exists between the survival times and the duration of DDW consumption by body weight in the study population cohort
3.2. Case studies of 4 pancreatic cancer patients
Below are detailed descriptions of four selected cases from the entire patient population. These cases are particularly notable because the patients began consuming DDW shortly after their diagnosis. Three of the patients underwent repeated DDW treatments, with breaks lasting a few months, and exhibited extended survival compared to typical pancreatic cancer patients.
3.2.1. Patient #1
A 68-year-old woman, weighing 65 kg, was diagnosed with pancreatic cancer during a routine abdominal ultrasound examination in August 2012. The histological classification of the inoperable tumor, located in the pancreatic body, was adenocarcinoma, measuring 26 mm. The patient began chemotherapy in November 2012. A follow-up CT scan in December 2012 revealed the tumor at 25 mm, with infiltration into the peripancreatic fat. By December 2013, the tumor had reduced slightly to 24 mm, and subsequent scans showed no significant changes. However, in February 2014, a hypodense area with a blurred contour, measuring 32 x 32 mm, was identified in the head of the pancreas, though it did not affect the surrounding adipose tissue. By February 2015, minimal regression was observed in both areas. The patient began consuming DDW in October 2012, alongside chemotherapy and continued using DDW until the end of 2015.
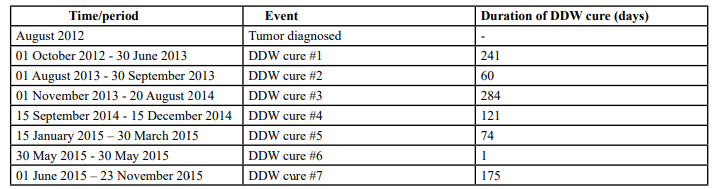
Table 1: The sequence of diagnosis events and DDW application in the case of Patient #1 in an overall period of 3 years (956 days). The period from diagnosis to the end of follow-up was 1,187 days. She was alive at the end of the observation period.
3.2.2. Patient #2
A 40-year-old woman weighing 60 kg was diagnosed in April 2009 with an inoperable pancreatic tumor. The cancer was histologically classified as adenocarcinoma, with multiple hepatic metastases. The patient underwent chemotherapy for three years. A CT scan on July 31, 2009, compared to the previous scan on April 28, 2009, showed partial regression in both the number and size of multiple hypodense liver lesions. The lesion in the left lobe decreased from 33 x 24 mm to 22 x 17 mm, while the lesion at the S.5-8 border shrank from 39 x 19 mm to 24 x 20 mm. The focus in S.4/B reduced from 42 x 31 mm to 26 x 23 mm, and the largest hypodense area at the pancreatic head-tail border decreased from 30 x 16 mm to 27 x 14 mm. Subsequent CT scans in the following years continued to show further regression.
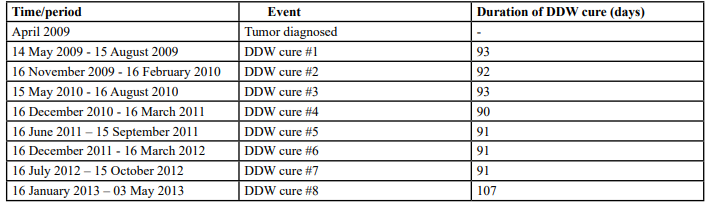
Table 2:The sequence of diagnosis events and DDW application in the case of Patient #2 in an overall period of 4 years (748 days). The period from diagnosis to the end of follow-up was 1,496 days.
3.2.3. Patient #3
A 44-year-old woman weighing 63 kg, was diagnosed with an inoperable pancreatic tumor during an exploratory laparotomy in April 2011. The tumor was histologically classified as adenocarcinoma. She underwent chemotherapy until April 2012 while also consuming DDW. By April 2012, control examinations showed tumor regression, but surgery remained unfeasible because the tumor could not be separated from the hepatic portal vein. Histological analysis revealed very few viable tumor cells at that time. In October 2012, a PET/CT scan no longer detected the tumor. In the spring of 2014, the patient underwent surgery due to tumor necrosis. Most of the tumor had calcified and was removed, with the remaining tissue identified as necrotic.
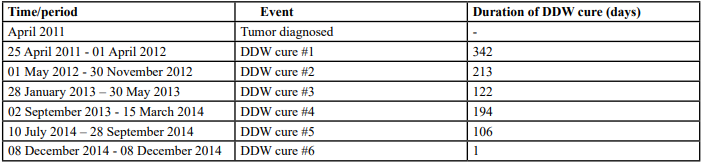
Table 3:: The sequence of diagnosis events and DDW application in the case of Patient #3 in an overall period of 3 years (978 days). The period from diagnosis to the end of follow-up was 1,343 days.
3.2.4. Patient #4
A 71-year-old man weighing 77 kg was diagnosed with pancreatic cancer in January 2003. The tumor, located in the head of the pancreas, measured 46 x 34 x 22 mm and was closely associated with the superior mesenteric artery, with slight infiltration of the surrounding fatty tissue. During exploratory surgery, the bile ducts were ligated, and a choledochojejunostomy was performed. In February 2003, the patient began chemotherapy. A CT scan in April 2004 showed no disease progression, and by June 2004, the tumor’s maximum diameter had reduced to 33 mm. However, an ultrasound in April 2004 measured the tumor at 54 x 49 x 38 mm. The patient continued consuming DDW throughout this period, he passed away in August 2004.

Table 4:: The sequence of diagnosis events and DDW application in the case of Patient #4 in an overall period of more than one year (493 days). The period from diagnosis to the end of follow-up was 588 days.
Discussion
Naturally occurring deuterium plays a critical role in cell growth, and its depletion has been shown to induce apoptosis in tumor cells, leading to partial or complete tumor regression [7,8,23]. In this retrospective study, data from 93 pancreatic cancer patients were analyzed. All patients used DDW in combination with conventional therapies. The study population was heterogeneous regarding disease stage at the start of DDW consumption, the time between diagnosis and the initiation of DDW treatment, the duration of DDW use, and the type and length of conventional therapies received. All patients had tumors, except for four whose tumors had been surgically removed earlier. The cumulative follow-up time from diagnosis was 128.1 years. Detailed case histories of four patients were provided separately, demonstrating the potential effectiveness of DDW as a supportive treatment.
The MST for the 93 patients in this study was 21.5 months from diagnosis and 16.8 months from the start of DDW consumption, representing a five- and fourfold increase in MST compared to historical controls [5,6,24]. This study demonstrated a twomonth longer median survival time (MST) compared to an earlier study involving 56 patients consuming deuteriumdepleted water (DDW), which reported an MST of 19.6 months [21]. The current findings confirm increased MST for female and male populations relative to the previous study (female: 37.5 vs. 21.4 months; male: 17.1 vs. 15.1 months), suggesting that cancer in female patients shows greater sensitivity to deuterium depletion. Similar trends have been observed in lung cancer and glioblastoma population studies [18,20]. This gender disparity may be explained by differences in oncogene expression levels and their response to deuterium depletion, as seen in female and male mice [13].
The timing of DDW treatment proved crucial in two key ways. First, longer DDW consumption was associated with extended survival, as indicated by the positive correlation between the duration of DDW use and survival time (Figure 3). Second, surprisingly, patients who began DDW consumption more than six months after diagnosis had a significantly longer MST (over 70 months) compared to those who started DDW within six months (17.1 months). A plausible explanation for this result is that many patients may have reached a stable condition due to conventional treatments lasting several months, allowing deuterium depletion to exert its antitumor effects more effectively from a better baseline health status. This finding highlights the importance of exploring optimal combinations of conventional cancer therapies and DDW treatment.
Another critical factor is the applied water’s D level (ppm value). Application always started with DDW of less depleted level of deuterium. However, for pancreatic cancer patients, one has to change for more and more D-depleted (lower ppm level) water in shorter intervals because this tumor is aggressive and grows very rapidly. However, it is difficult for the body to clear away the necrotized tumor so that an abscess can form.
In light of scientific evidence supporting the anticancer effect of deuterium depletion [8,14,23,25], the observed positive correlation between the duration of DDW consumption and survival (Figure 3) provides further support for DDW’s anticancer efficacy in pancreatic cancer patients. Prolonged DDW consumption was associated with longer survival times. Interestingly, there was no statistically significant correlation between body weight and the duration of DDW consumption, contrary to earlier observations from over 2,000 DDWconsuming cancer patients.
In the four detailed cases, significantly longer survival times were observed than previously published data [5,6,24]. This provides further evidence that deuterium depletion, when used alongside conventional treatments, can extend survival in advanced-stage pancreatic cancer more effectively than targeted or combination therapies alone.
Oral DDW treatment is safe and non-toxic. Preclinical toxicology studies [26], along with both prospective and retrospective clinical trials [15,16,18,20,21], have consistently demonstrated that no adverse effects or unwanted events occurred during long-term DDW use across a wide concentration range (125 to 25 ppm). It is important to note that cancer is not the only indication where deuterium depletion has well-established benefits. Studies in rats and human clinical trials indicate that deuterium concentration substantially impacts metabolic processes. Specifically, maintaining deuterium levels within the 125–140 ppm range optimizes insulin signaling, reduces fasting glucose levels, and improves insulin resistance [27,28]. Additionally, other research has highlighted the anti-aging benefits of deuterium depletion [29].
In conclusion, deuterium depletion offers a valuable addition to conventional therapies and can be effectively integrated into standard treatment protocols for pancreatic cancer patients. Despite the inherent limitations of the retrospective approach, our findings may support the need for and feasibility of prospective clinical trials to further evaluate DDW’s potential benefits.
Funding Sources
This work was sponsored by HYD LLC for Cancer Research and Drug Development.
Declaration of Conflicting Interests
The authors declare no conflict of interest.
References
1. Davis, J.L., Pandalai, P.K., Ripley, R.T., Langan, R.C., Avital, I. (2012). Expanding surgical treatment of pancreatic cancer: the role of regional chemotherapy. Pancreas, 41(5), 678-684.
2. Lim, J., Chien, M., Earle, C. (2003). Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann Surg, 237, 74-85.
3. Trade, M., Schwal, G., Saeger, H. (1990). Survival after pancreatoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg, 211, 447-458.
4. Geer, R., Brennan, M. (1993). Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Ann Surg, 165, 68-72.
5. Burris, H.A., Moore, M.J., Andersen, J., et al. (1997). Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol, 15, 2403- 2413.
6. O’Reilly E.M. (2013). Adjuvant therapy for pancreas adenocarcinoma. J Surg Oncol, 107(1), 78-85.
7. Somlyai, G., Laskay, G., Berkényi, T., Jákli, Gy., Jancsó, G. (1998). Naturally occurring deuterium may have a central role in cell signaling. In Synthesis and Applications of Isotopically Labelled Compounds. Heys JR, Melillo D. Eds, John Wiley and Sons Ltd: New York, USA, 137–141.
8. Somlyai, G., Jancsó, G., Jákli, G., Vass, K., Barna, B., et al. (1993). Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett, 317(1-2), 1.
9. Somlyai, G., Laskay, G., Berkényi, T., Galbács, Z., Galbács, G., et al. (1998). The biological effects of deuteriumdepleted water a possible new tool in cancer therapy. Journal of Oncology, 30, 91-94. 10. Yavari, K., Kooshesh, L. (2019). Deuterium Depleted Water Inhibits the Proliferation of Human MCF7 Breast Cancer Cell Lines by Inducing Cell Cycle Arrest. Nutrition and Cancer, 71(6), 1019-1029.
11. Syroeshkin, A., Levitskaya, O., Uspenskaya, E., Pleteneva, T., Romaykina, D, et al. (2019). Deuterium Depleted Water as an Adjuvant in Treatment of Cancer. Sys Rev Pharm, 10(1), 112-117.
12. Zlatskiy, I.A., Zlatska, A.V., Antipova, N.V., Syroeshkin, A.V. (2018). Effect of deuterium on the morpho-functional characteristics of normal and cancer cells in vitro. Trace Elements and Electrolytes, 35(4), 211-214.
13. Gyöngyi, Z., Somlyai, G. (2000). Deuterium depletion can decrease the expression of C-myc Ha-ras and p53 gene in carcinogen-treated mice. In Vivo, 14:437-439.
14. Zhang, X., Gaetani, M., Chernobrovkin, A., Zubarev, R.A. (2019). Anticancer Effect of Deuterium Depleted Water – Redox Disbalance Leads to Oxidative Stress. Mol Cell Proteomics, 18(12), 2373-2387.
15. Kovács, A., Guller, I., Krempels, K., Somlyai, I., Jánosi, I., et al. (2011). Deuterium depletion may delay the progression of prostate cancer. J Cancer Ther, 2, 548-556.
16. Krempels, K., Somlyai, I., Gyöngyi, Z., Ember, I., Balog, K., et al. (2013). A retrospective study of survival in breast cancer patients undergoing deuterium depletion in addition to conventional therapies. Journal of Cancer Research & Therapy, 1(8), 194-200.
17. Gyöngyi, Z., Budán, F., Szabó, I., Ember, I., Kiss, I., et al. (2013). Deuterium Depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras, Bcl2, and Myc Genes in Mouse Lung. Nutrition and Cancer. 2013.
18. Somlyai, G., Kovács, B.Zs., Somlyai, I., Papp, A., Nagy, L.I., Puskás, L.G. (2021). Deuterium depletion inhibits lung cancer cell growth and migration in vitro and results in severalfold increase of median survival time of non-small cell lung cancer patients receiving conventional therapy. Journal of Cancer Research & Therapy, 9(2), 12-19.
19. Kovács, B.Zs., Puskás, L.G., Nagy, L.I., Papp, A., Gyöngyi, Z., Fórizs, I., Czuppon, Gy., Somlyai, I., Somlyai, G. (2022). Blocking the Increase of Intracellular Deuterium Concentration Prevents the Expression of Cancer-Related Genes, Tumor Development, and Tumor Recurrence in Cancer Patients. Cancer Control, 29, 1–11.
20. Somlyai, G., Kovács, B.Zs., Papp, A., Somlyai, I. (2023). A Preliminary Study Indicating Improvement in the Median Survival Time of Glioblastoma Multiforme Patients by the Application of Deuterium Depletion in Combination with Conventional Therapy. Biomedicines, 11, 1989.
21. Boros, L.G., Somlyai, I., Kovács, B.Zs., Puskás, L.G., Nagy, L.I.., Dux, L., Farkas, Gy., Somlyai, G. (2021). Deuterium Depletion Inhibits Cell Proliferation, RNA and Nuclear Membrane Turnover to Enhance Survival in Pancreatic Cancer. Cancer Control, 8, 1-12.
22. London, H. (1961). Separation of isotopes. George Newnes Limited: London, England; 1961.
23. Cong, F.S., Zhang, Y.R., Sheng, H.C., Ao, Z.H., Zhang, S.Y., et al. (2010). Deuterium-Depleted Water Inhibits Human Lung Carcinoma Cell Growth by Apoptosis. Exp. Ther. Med, 1(2), 277.
24. Miller, R.C., Iott, M.J., Corsini, M.M. (2009). Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys, 75(2), 364-368.
25. Kamal, Y., Lida, K. (2019). Deuterium Depleted Water Inhibits the Proliferation of Human MCF7 Breast Cancer Cell Lines by Inducing Cell Cycle Arrest. Nutrition and Cancer, 71(6), 1019-1029.
26. Török, G., Csík, M., Pintér, A. (2000). Effects of Different Deuterium Concentrations of the Media on the Bacterial Growth and Mutagenesis. Egészségtudomány/Health Science, 44, 331.
27. Somlyai, G., Somlyai, I., Fórizs, I., Czuppon, Gy., Papp, A., Molnár, M. (2020). Effect of Systemic Subnormal Deuterium Level on Metabolic Syndrome Related and other Blood Parameters in Humans: A Preliminary Study. Molecules, 25, 1376.
28. Molnár, M., Horváth, K., Dankó, T., Somlyai, I., Kovács, B.Zs., Somlyai, G. (2021). Deuterium-depleted water stimulates GLUT4 translocation in the presence of insulin, which leads to decreased blood glucose concentration. Molecular and Cellular Biochemistry, 476, 4507–4516.
29. Ávila, D.S., Somlyai, G., Somlyai, I., Aschner, M. (2012). Anti-aging effects of deuterium depletion on Mn-induced toxicity in a C. elegans model. Toxicology Letters, 211, 319- 324.



