Case Report - (2024) Volume 9, Issue 1
Bladder Decompression as a Non-Renal Replacement Intervention for Acute Renal Failure and Severe Hyperkalemia with EKG Changes in Obstructive Nephropathy
2Department of Nephrology and Hypertension, Cleveland Clinic Lerner College of Medicine, Case Western, USA
Received Date: Dec 26, 2023 / Accepted Date: Jan 04, 2024 / Published Date: Jan 15, 2024
Copyright: ©2024 Sushil K Mehandru, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Citation: Oppegaard, K., Kaur, S., Masud, A., Dandu, A., & Perez, J., et al. (2024). Bladder Decompression as a Non-Renal Re-placement Intervention for Acute Renal Failure and Severe Hyperkalemia with EKG Changes in Obstructive Nephropathy. J Clin Rev Case Rep, 9(1), 332-338.
Abstract
Renal replacement therapy is indicated in cases of acute renal failure and hyperkalemia with EKG changes regardless of etiology. In this presentation, we report seven cases of obstructive nephropathy resulting in acute renal failure and hyperkalemia with EKG changes, successfully managed through bladder decompression using a foley catheter and a potassium-lowering protocol. We aim to highlight the clinical utility of bladder decompression as a promising intervention for management of acute renal failure and hyperkalemia causing EKG changes in patients with obstructive nephropathy. This is the first case report in the literature to demonstrate such to the best of our knowledge. Existing literature has shown the absence of immediate relief in obstructive nephropathy can result in permanent renal damage, influencing clinicians to initiate renal replacement therapy. Our team utilized bladder decompression during a typical 2-hour window that would take to initiate renal replacement therapy to actively reduce serum potassium levels, reverse EKG abnormalities, and improve renal function. By presenting these cases, we hope to provide clinicians with a broader perspective for the management of hyperkalemia and acute renal failure in obstructive nephropathy without the need of renal replacement therapy.
Keywords
Hyperkalemia, EKG change, AKI, Obstructive nephropathy, Bladder drainage
Introduction
Acute renal failure and hyperkalemia are critical conditions requiring prompt intervention to avert any potential lifethreatening complications. This case series presentation sheds light on a distinct approach of bladder decompression through the use of a foley catheter coupled with a potassium-lowering protocol. Within 2-4 hours, the amount of time it would conventionally take to initiate dialysis, we were able to observe improvement in serum potassium levels as well as renal function along with immediate reversal of EKG changes. We present seven cases where this unconventional intervention yielded successful outcomes. By doing so, we aim to underscore the clinical utility of bladder decompression as an effective strategy in addressing acute renal failure and hyperkalemia with EKG changes in patients with obstructive nephropathy.
Obstructive Nephropathy comprises 5-10% of acute kidney injury cases [1] and potentially escalating to 22% in the elderly population [2], encompasses a spectrum of causative factors, as illustrated in Table 1. Notably in our observation, prostate enlargement, bladder carcinoma, and bladder stones are the major causes of obstructive nephropathy in males as shown in Table 3. After bladder decompression with a foley catheter, serum potassium, blood urea nitrogen (BUN), creatinine and serum bicarbonate (HCO3) levels were monitored every 2 hours. The sequelae of obstructive nephropathy can precipitate acute kidney injury, often resulting in the development of hyperkalemia with associated EKG changes, a clinical scenario prompting consideration of urgent renal replacement therapy. It is worth highlighting that the recommended initial approach for patients facing acute hyperkalemia with EKG changes involves the stabilization of the cardiac membrane [3]. EKG changes at various potassium levels are explored in Table 2. In our clinical context, this objective was successfully achieved through the implementation of our potassium-lowering protocol and bladder drainage. In lieu of resorting to immediate renal replacement therapy, our approach was focused on the strategic utilization of bladder decompression, as an alternative means to mitigate acute kidney injury and rectify hyperkalemia with EKG changes. All our patients, except for case #3 and case #5 had normal renal function prior to presentation and had complete resolution of acute kidney injury. There was previous kidney disease present in case #3 and case #5, and therefore we did not see complete resolution of renal function, but we did observe improvement of serum potassium, resolution of EKG changes and improvement of serum creatinine and BUN to the patient’s baseline. This approach of bladder decompression and a potassium lowering protocol represents a noteworthy departure from conventional urgent renal replacement therapy and forms the core of our presentation. The process also increases hemodynamic stability and avoidance of vas-catheter associated infection that can often lead to sepsis.
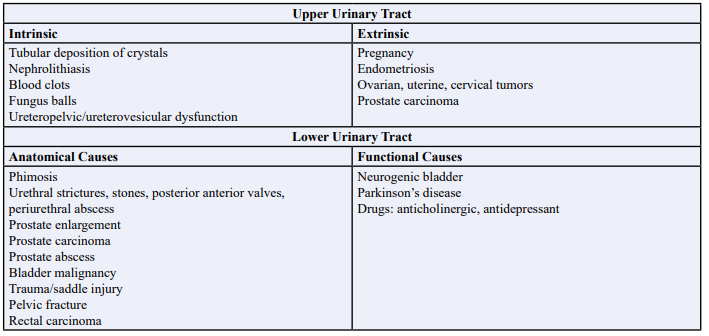
Table 1: Causes of Obstructive Nephropathy: Upper Urinary Tract and Lower Urinary Tract.

Table 2: EKG Changes with Hyperkalemia.
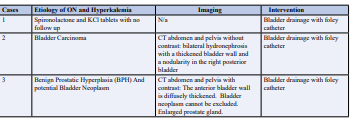
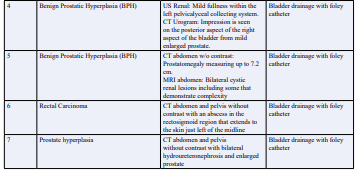
Table 3: Etiology of Obstructive Nephropathy (ON) and Hyperkalemia in our Cases.
Case Report
2.1 Case 1
55-year-old male with no previous history of renal disease, was referred to our hospital by his cardiologist for shortness of breath and an abnormal outpatient EKG. On admission, the patient was found to have a serum creatinine of 5.84 mg/dL, blood urea nitrogen 21 mg/dL, eGFR 11 cc/min, serum potassium 7.3 mEq/L, and bicarbonate less than 10 mEq/L. EKG revealed disappearance of P wave and peak T wave. Patient endorsed that he has experienced decreased urine output over the past 3 weeks. Bladder drainage was achieved through foley catheter insertion, which drained more than 1000 ml of urine immediately and a potassium lowering protocol was administered. Within 2 hours, hyperkalemia induced EKG changes were resolved. Repeat labs within 4 hours revealed improved potassium to 5.9 mEq/L, creatinine 5.65 mg/dL, BUN 88 mEq/L, eGFR 16 cc/min, bicarbonate 11 mEq/L. Within 24 hours, labs showed further improvement with potassium of 4.7 mEq/L, creatinine 3.41 mg/dL, BUN 80 mEq/L, eGFR 20 cc/min and bicarbonate 17 mEq/L. Within one week of AKI, GFR returned to normal limits of >60 cc/min.
2.2 Case 2
A 73 year old male with no prior history of renal dysfunction presented to the emergency department with disorientation and a change in mental status. The patient endorsed urinary retention, with creatinine of 7.34 mg/dL, eGFR 7 cc/min, bicarbonate 18 mEq/L, BUN 164 mEq/L and serum potassium of 7.6 mEq/L, causing acute EKG changes (Figure 1). Potassium lowering protocol was initiated and a foley catheter was inserted with 1.5 L of bloody urine output. Within 2 hours, hyperkalemia induced EKG changes had resolved (Figure 2), serum potassium was near normal at 5.1 mEq/L, creatinine was 6.12 mg/dL, BUN 149 mEq/L, eGFR 9 cc/min, and bicarbonate 25 mEq/L. Within 24 hours of bladder drainage, serum potassium levels were 4.6 mEq/L with creatinine of 2.89 mg/dL, BUN 129 mEq/L, eGFR 40 cc/min, and bicarbonate 23 mEq/L. Within 48 hours of bladder drainage further improvement was noted, serum potassium, creatinine, BUN, GFR and bicarbonate levels were noted to be near normal with potassium of 4.1 mEq/L, creatinine 1.3 mg/ dL, BUN 33 mEq/L, eGFR>60 cc/min, and bicarbonate of 27 mEq/L. We were able to successfully treat acute renal failure and severe hyperkalemia with potassium lowering protocol and bladder decompression.
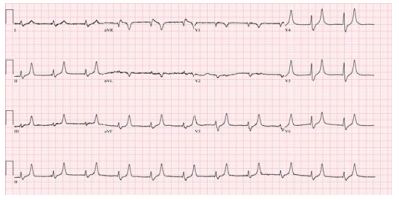
Figure 1: Peaked T waves due to hyperkalemia seen in Case #2
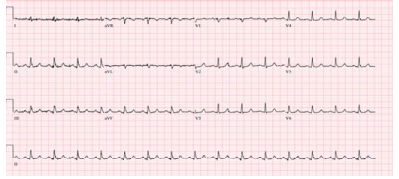
Figure 2: Resolution of EKG changes in Case #2 within 4 hours after foley catheter insertion and potassium lowering protocol.
2.3 Case 3
An 84 year old male with a history of chronic kidney disease, and benign prostatic hyperplasia (BPH), presented to the emergency department after being advised by his primary care provider to seek medical attention for further evaluation of elevated creatinine and potassium levels. He was found to have a creatinine of 5.92 mg/dL, potassium 5.7 mEq/L, BUN 92 mEq/L, eGFR 9 cc/ min, and bicarbonate 13 mEq/L. EKG showed peak T-waves and prolonged PR interval. CT scan of the abdomen and pelvis revealed bilateral hydronephrosis and hydroureter, diffuse bladder wall thickening and an enlarged prostate. Bladder neck obstruction at the prostate level resulted in acute hydronephrosis and AKI on CKD. Bladder drainage was achieved with a foley catheter with an output of 1500 mL of urine, and potassium lowering protocol. Potassium levels improved and normalized to 4.5 mEq/L and a reversal of EKG changes was noted. Patient’s creatinine improved to 2.87 mg/dL (which is the patient’s baseline) BUN to 77 mEq/L, eGFR to 26 cc/min, and bicarbonate to 17 mEq/L. It is important to note that this patient had a history of CKD prior to presentation. Consequently, although serum creatinine, BUN, and GFR levels did not fully normalize, they did revert to the patient’s baseline values.
2.4 Case 4
70-year-old male with a medical history of metastatic renal cell carcinoma, presented to the ED for altered mental status, generalized weakness, and bilateral lower extremity edema. In the ED, the patient was awake, alert, and oriented X 1, with stable vitals. Labs were significant for BUN 146 mEq/L, creatinine 12.49 mg/dL, eGFR 4 cc/min, bicarbonate 11 mEq/L, and serum potassium of 8.8 mEq/L. EKG was remarkable for widened QRS complexes with peaked T waves. Bladder and renal ultrasound confirmed that the patient had an acute kidney injury due to bladder neck obstruction secondary to BPH. Potassium lowering protocol was administered and bladder drainage with a foley catheter resulted in drainage of 1500 cc of clear urine within 90 mins. EKG changes were reversed without initiation of renal replacement therapy within 2 hours. After 24 hours of foley catheter placement, the patient’s creatinine significantly improved to 1.26 mg/dL, eGFR at 60 cc/min, BUN 22 mEq/L, potassium 4.6 mEq/L, and bicarbonate to 25 mEq/L. The patient’s acute kidney injury and hyperkalemia with EKG changes secondary to obstructive nephropathy completely resolved with bladder drainage and potassium lowering protocol.
2.4 Case 5
79-year-old male with a past medical history of benign prostatic hyperplasia and underlying chronic kidney disease, presented to the emergency department with lethargy, shortness of breath and decreased urine output. He was found to have creatinine 3.52 mg/ dl with an eGFR 17 cc/min, a BUN 84 mEq/L, potassium 6.1 mEq/L, and bicarbonate 22 mEq/L. EKG showed prolonged QRS, peaked T-waves and complete heart block. Potassium lowering protocol was administered in addition to intermittent bladder drainage with a foley catheter insertion that resulted in drainage of 4200 cc of urine in the first two hours. EKG changes had resolved. After 72 hours of presentation, patient’s potassium was stable at 4.4 mEq/L. One week after admission, his eGFR was 21 cc/min, creatinine had fallen to 2.92 mg/dl, BUN 70 mEq/L, and bicarb was 25 mEq/L, and his potassium remained stable. It is important to note that this patient had history of CKD prior to presentation, resulting in creatinine, BUN, and GFR to his baseline.
2.5 Case 6
A 51-year-old male with a past medical history of advanced rectal cancer, was admitted to the hospital with acute renal failure secondary to obstructive nephropathy. The patient was unable to urinate for 3 days and had associated lower extremity and scrotal swelling. On admission to the hospital, serum creatinine was 11.18 mg/dL, eGFR 6 cc/min, BUN 96 mEq/L, bicarbonate of 15 mEq/L, and serum potassium was 5.6 mEq/L. EKG showed peaked T waves. Patient had intermittent bladder drainage with a foley catheter, resulting in drainage of 2L of urine and was administered potassium lowering protocol. Within 2 hours, serum creatinine was 9.11 mg/dL, eGFR 6 cc/min, BUN 61 mEq/L, bicarbonate 18 mEq/L, and serum potassium 4.5 mEq/L. EKG changes resolved. Over the next 48 hours his GFR returned to normal, >60 cc/min with improvement in BUN and electrolytes.
2.6 Case 7
77-year-old male with a past medical history of hypertension, hyperlipidemia, benign prostatic hyperplasia, presented from home with lower abdominal pain and difficulty urinating for the past 5 days. Patient was admitted to the hospital for acute renal failure due to obstructive nephropathy from an enlarged prostate. On admission, the patient had a serum creatinine of 4.3 mg/dL, BUN 65 mEq/L, eGFR 14 cc/min, serum bicarbonate 21 mEq/L, and serum potassium 6.2 mEq/L. EKG showed peaked T waves. Patient had over 1500 cc of urine drained from his bladder with a foley catheter and received potassium lowering protocol. Within 2 hours, serum creatinine improved to 2.47 mg/dL, BUN 45 mEq/L, eGFR to 26 cc/min, bicarbonate 23 mEq/L, and serum potassium to 4.9 mEq/L. EKG changes resolved. A week after AKI, his renal function improved to eGFR >60 cc/min and electrolytes were normal.
Discussion
Obstructive nephropathy should always be included as a part of differential diagnosis in treatment of acute renal injury, especially in presence of a sudden decrease in urine output. Obstructive nephropathy refers to a disorder of the urinary tract that occurs due to blockage of urinary flow and can be either structural or functional. The back-up of urine into the unilateral or bilateral kidneys, depending on the location of the obstruction, can be complete or partial. [4] “When there is a restriction to the normal flow of urine through the urinary tract, there will be a back pressure of urine into the collecting system of the kidneys. This built up pressure may cause hydronephrosis. In time, this may produce dilatation within the tract, and, as the kidney filtration system is affected, becoming the primary reason for the development of obstructive nephropathy” [4]. “Partial chronic obstruction of the urinary tract can lead to progressive atrophy and destruction of nephrons may result in chronic renal insufficiency. On the other hand, complete obstruction occurring suddenly results in acute renal failure. Because obstruction generally is a remediable cause of kidney failure, early and accurate diagnosis and prompt implementation of appropriate therapeutic maneuvers assume considerable importance in the preservation and restoration of renal function” [5]. During our encounter with patients presenting with obstructive nephropathy causing AKI, insertion of foley catheter and implementation of potassium lowering protocol resulted in reversal of EKG abnormalities and improvement in AKI within the same or reduced time frame that would be required for initiation of renal replacement therapy. This reduced risk of catheter induced infection as well as maintained hemodynamic stability often associated with hemodialysis.
“An increase in the intraluminal pressure of the ureter and renal tubules is one of the earliest detectable effects of urinary tract obstruction. The rate of increase of ureteral and intratubular pressure depends on the rate of urine flow at the onset of obstruction. In rats undergoing mannitol diuresis, ureteral and intratubular pressures reached values of approximately 40 mm Hg within 10 to 15 minutes after the onset of obstruction. In animals not receiving diuretics, ureteral pressure rose to approximately 14 mm Hg, and intratubular pressure did not increase at all during this time interval. When dogs were subjected to ureteral obstruction during saline or mannitol diuresis, ureteral pressure reached 50 to 150 mm Hg within 5 to 15 minutes after the onset of obstruction. If the obstruction persists for more than 4 hours, the intratubular pressure begins to decline; if only one ureter is obstructed, intratubular pressure returns to nearly normal (9-15 mm Hg) after 24 hours of obstruction. This decline in intratubular pressure can result from a reduction of fluid volume in the tubular system because of decreased glomerular filtration, increased tubular reabsorption, increased capacity of a compliant pelvis, or some combination of these factors” [5]. “When both ureters are obstructed in experimental animals, intratubular pressure increases to higher levels than when only one ureter is obstructed. In addition, in bilateral ureteral obstruction, the subsequent decline in intratubular pressure does not return the values to normal. Intratubular pressure has been found to be 30 mm Hg, or about 3 times normal values, after bilateral obstruction for 24 hours in rats. Ureteral pressure also remains higher after 24 hours of bilateral ureteral obstruction than after the same period of unilateral obstruction” [5] “The rate of glomerular filtration is governed by: (1) net ultrafiltration pressure across glomerular capillaries; (2) permeability of the glomerular capillary wall to water and small solutes; and (3) total surface area of the capillaries” [5] “Theoretically, urinary tract obstruction can decrease GFR by affecting one or more of the factors that govern the rate of ultrafiltration across glomerular capillaries. Few studies are available on the effects of continuing obstruction on glomerular filtration rate in humans” [5]. “Wright measured the so-called proximal tubule stop-flow pressure, an indirect index of glomerular capillary pressure, following the onset of unilateral or bilateral ureteral obstruction in rats [6]. As flow stops and glomerular filtration falls to zero, the tubule pressure becomes the difference between glomerular capillary hydrostatic pressure and oncotic pressure” [6].
“The increase in glomerular capillary pressure during the first few hours after the onset of obstruction suggests a decrease in preglomerular resistance, an increase in post-glomerular resistance, or a combination of both. An increase in postglomerular resistance would result in a decrease or no change in renal blood flow if pre-glomerular resistance remains the same. Numerous studies indicate that renal blood flow through the obstructed kidney increases during the first few hours following the onset of obstruction. The increase in renal blood flow caused by ureteral obstruction appears limited to the renal cortex. Inner medullary plasma flow decreases from the onset of ureteral obstruction, reaching values about 30% of normal within 6 hours” [6] “When we consider the forces responsible for ultrafiltration across the glomerular capillaries, it becomes clear that the effects of acute obstruction in decreasing glomerular filtration rate initially arise mainly from an increase in intratubular pressure and hence a decrease in effective filtration pressure (the difference between intraglomerular capillary pressure and the pressure in Bowman’s space and/or intratubular pressure). Because, at least in the case of unilateral ureteral obstruction, intratubular pressures and hence pressures in Bowman’s space return to normal levels within 24 hours, the decrease in effective ultrafiltration pressure during that period is mainly due to a decrease in hydrostatic pressure in glomerular capillaries’’ [6] “This data suggests that progressive preglomerular constriction occurs as obstruction persists for more than 5 hours. Various groups have observed that obstruction for 24 hours results in a decrease in total glomerular filtration rate of approximately 80% immediately after the obstruction is relieved. Measurements of single-nephron GFR indicates, however, that in surface nephrons, single nephron GFR decreases by only 60% to 70%, whereas single nephron GFR in the juxtamedullary units decreases by approximately 50%. This data suggests that there are filtering and non-filtering nephrons during this period of obstruction, with filtering nephrons exhibiting a decrease in single-nephron GFR” [5]. “Values for creatinine clearance, which were markedly decreased in the immediate post-release period, returned to normal approximately one week after the relief of urethral obstruction and were comparable with those obtained in a group of sham-operated rats. These studies thus indicate that the decline in GFR observed with short-term obstruction is completely reversible and that most of the early alterations are functional in nature and do not involve permanent loss of nephrons. By contrast, complete long-term obstruction (more than one week), even if released, leads to permanent loss of renal function, which is greater as the period of obstruction is lengthened” [5]. “Nevertheless, glomerular capillary hydrostatic pressure was significantly higher in obstructed than in non-obstructed kidneys, whereas values for intratubular pressure were similar” [5]. These points are demonstrated in cases #3 and case #5, where long term obstruction in patients with previous renal dysfunction had GFR that remained lower one week after bladder decompression. In comparison, case #2 had obstruction for much longer than case #5, which resulted in bilateral hydronephrosis on renal ultrasound, and in case #5, the patient had a benign renal ultrasound.
“The renal hemodynamic alterations during acute or chronic urinary tract obstruction contribute significantly to the pathogenesis of obstructive nephropathy. During the first phase, renal blood flow actually rises above control values and is associated with a decline in intrarenal resistance. This increase in blood flow occurs during the first 1 to 2 hours after obstruction and is associated with gradually increasing ureteral pressure. In the second phase, approximately 2 to 5 hours after obstruction, renal blood flow declines, returning to control values, while ureteral pressure continues to increase. Finally, in the third phase, ureteral pressure begins to return to control values, but renal blood flow continues to decline and falls progressively with time” [5].
Potassium is the second most abundant cation in the body and plays a vital role in various essential physiological processes, including cellular metabolism, glycogen and protein synthesis and the crucial management of electrical action potential across cell membranes, particularly within the myocardium [7]. According to the National Institute of Health, the upper limit of normal for potassium is 5.0mEq/L, and hyperkalemia is defined as a potassium level of 5.5 mEq/L or above [8]. “The normal kidney has a large capacity to excrete potassium [8]. Accumulation of potassium in the interstitium after increased intake exerts an inhibitory effect on the thick ascending limb and to a lesser extent, proximal tubular sodium chloride reabsorption, resulting in increased flow and sodium delivery to the distal nephron. High potassium intake modulates flow and sodium delivery through direct effects in the distal convoluted tubule. Elevations in plasma potassium are registered by cells in the initial portion of this segment, decreasing activity of the thiazide-sensitive sodium chloride cotransporter. In consequence, flow and sodium are delivered increasingly to the adjacent aldosterone sensitive distal nephron, where electrogenic and flow-mediated potassium secretion is enhanced” [9]. “Hyperkalemia is a common complication of AKI when the injury involves the late distal nephron and extends into the collecting duct, causing direct injury of cells responsible for potassium secretion. Such injury can result from acute tubular necrosis due to ischemia or toxins or from inflammation as in acute tubulointerstitial nephritis. Hyperkalemia is an early finding in acute urinary obstruction because increased tubular pressure disrupts the high resistance nature of the distal nephron, leading to loss of the electrical driving force for potassium secretion. Sudden reductions in the GFR become a limiting factor for potassium secretion in patients with AKI. In patients with oliguria, reduced distal delivery of salt and water further contributes to decreased distal potassium secretion [9].
Hyperkalemic metabolic acidosis has been recognized as a sequela of obstructive nephropathy. The pathogenesis of this is a defect in potassium and hydrogen ion excretion in the distal renal tubules leading to a distal renal tubular acidosis. This hyperkalemic acidosis that develops in some patients may be due to a decrease in aldosterone occurring due to damage to the kidney interstitium leading to decreased renin release. This is common in elderly patients with obstructive uropathy who often present with both renin and aldosterone deficiencies [3]. Other causes include Addison’s disease, potassium chloride administration, and administration of potassium sparing diuretics or prostaglandin inhibitors. However, it has been shown that hyperkalemic metabolic acidosis in the setting of obstructive nephropathy still occurs in some patients despite appropriate levels of aldosterone. A study done by Battle et al., they characterized patients with hyperkalemic distal renal tubular acidosis in patients with obstructive nephropathy due to aldosterone deficiency differed from those with adequate levels of plasma aldosterone due to their ability to lower urinary pH below 5.5 during metabolic acidosis. The study tested the patient’s ability to increase potassium excretion with delivery of sodium sulfate, a compound known to enhance potassium excretion by increasing trans-tubular electrical gradient. In the subset of patients with adequate levels of aldosterone, administration of sodium sulfate after pretreatment with fludrocortisone did not increase potassium excretion to normal and urinary pH did not fall below 5.5, suggesting inadequate mineralocorticoid activity. However, marked fall in urinary pH was observed in normal subjects, patients with chronic renal failure, and patients with aldosterone deficiency (fludrocortisone only) given sodium sulfate. Similar to the patients with obstructive nephropathy, this exact pattern of electrolyte excretion has also been demonstrated in animals treated with amiloride [3], suggesting that inadequate sodium reabsorption in the distal tubule leading to voltage dependent defect may be preventing appropriate excretion of potassium and hydrogen ions despite appropriate aldosterone levels [3]. Regardless of the mechanism, obstructive nephropathy was present along with hyperkalemic acidosis in each of the cases demonstrated in our case series and was appropriately resolved with the proposed treatment of hyperkalemia protocol and urinary bladder drainage.
Conclusion
Our team utilized the 2-hour window that it would take to initiate renal replacement therapy to actively reduce serum potassium levels, reverse EKG abnormalities, and improve acute kidney injury. In presenting our findings, we aim to highlight a novel perspective in the realm of obstructive nephropathy management. Our case series emphasizes the efficacy of foley catheter insertion as a promising intervention for addressing acute renal failure and hyperkalemia with EKG changes within the context of obstructive nephropathy. As far as our collective knowledge extends, there is an absence of documented case series illustrating the utilization of bladder drainage as a therapeutic strategy in addressing obstructive nephropathy, acute renal failure, and hyperkalemia with EKG changes. This approach of employing foley catheter insertion for bladder drainage can eliminate the need for renal replacement therapy in obstructive nephropathy.
Conflict of Interest Statement
None
Acknowledgement
None.
Informed Consent
Informed consent was obtained from patients.
Data Availability
The data supporting the findings of this study are available from the corresponding author.
Funding Sources
None.
References
1. Chávez-Iñiguez, J. S., Navarro-Gallardo, G. J., MedinaGonzález, R., Alcantar-Vallin, L., & García-García, G. (2020). Acute Kidney Injury Caused by Obstructive Nephropathy. International journal of nephrology, 2020, 8846622.
2. Dépret, F., Peacock, W. F., Liu, K. D., Rafique, Z., Rossignol, P., & Legrand, M. (2019). Management of hyperkalemia in the acutely ill patient. Annals of intensive care, 9(1), 32.
3. Batlle, D.C., Mozes, M.F., Manaligod, J., Arruda, J.A., Kurtzman, N.A. (1981).The pathogenesis of hyperchloremic metabolic acidosis associated with kidney transplantation. Am J Med 70(4), 786-796.
4. KERR, W.S Jr. (1956). Effects of complete ureteral obstruction in dogs on kidney function. Am J Physiol, 184(3), 521-526.
5. Klahr, S. (1983). Pathophysiology of obstructive nephropathy. Kidney Int 23(2), 414-426.
6. WRIGHT, F.S. (1982). Effects of urinary tract obstruction on glomerular filtration rate and renal blood flow, in Obstructive Uropathy, edited by KLAHR 5, New York. Grune & Stratton Inc. 1982, 5-16.
7. Chaitman, M., Dixit, D., & Bridgeman, M.B. (2016). Potassium-Binding Agents for the Clinical Management of Hyperkalemia. P & T: J for Formulary Management, 41(1), 43-50.
8. Simon, L.V., Hashmi, M.F., Farrell, M.W. (2023). Hyperkalemia. (Updated 2023 Feb 19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
9. Palmer, B.F., & Clegg, D.J. (2018). Hyperkalemia across the Continuum of Kidney Function. Clinical J of American Society of Nephrology: CJASN, 13(1), 155-157.



