Case Report - (2023) Volume 8, Issue 6
Baclofen as a Cause of Impaired Consciousness in a Haemodialysis Patient: A Case Report and Literature Review
2Brighton and Sussex Medical School, Brighton, UK
Received Date: May 27, 2023 / Accepted Date: Jun 06, 2023 / Published Date: Jun 10, 2023
Abstract
Baclofen is an agonist of the gamma-aminobutyric acid (GABA) receptor found on both the pre-and postsynaptic neurones in the central and peripheral nervous systems [1]. Binding to GABAB receptors reduces the activation of the neuronal action potentials supplying muscle spindles, resulting in muscle relaxation [2]. This effect is used therapeutically to treat muscle spasticity in conditions such as multiple sclerosis [3]. In addition, the side effects of baclofen, including dizziness and drowsiness, are thought to result from the activation of GABA receptors located in the central and sympathetic nervous systems [2].
Keywords
Baclofen toxicity, Serum inflammatory markers, Muscle relaxation, Dialysis, Haemofiltration, Multiple sclerosis
Introduction
Baclofen is an agonist of the gamma-aminobutyric acid (GABA) receptor found on both the pre-and postsynaptic neurones in the central and peripheral nervous systems [1]. Binding to GABAB receptors reduces the activation of the neuronal action potentials supplying muscle spindles, resulting in muscle relaxation [2]. This effect is used therapeutically to treat muscle spasticity in conditions such as multiple sclerosis [3]. In addition, the side effects of baclofen, including dizziness and drowsiness, are thought to result from the activation of GABA receptors located in the central and sympathetic nervous systems [2].
Around 80% of baclofen is excreted unchanged in urine through renal elimination. It relies mainly on glomerular filtration [4]. Therefore, the risk of baclofen toxicity has been demonstrated in patients with renal impairment, even with initiation on low baclofen doses [5]. Both continuous veno-venous haemofiltration [6] and haemodialysis [7] have been recognised as treatment options for baclofen toxicity. On the converse, there are only a few reports of baclofen toxicity in haemodialysis patients. Starting baclofen at a low dose and careful monitoring for toxicity are two crucial points if it is used in haemodialysis patients. We present a case of a haemodialysis patient presented with baclofen toxicity.
Case Report
A 75-year-old woman had increased drowsiness and dyskinetic muscle twitches with hypotension while on a typical in-centre haemodialysis session. She was admitted to the renal ward for investigation. In the days preceding this admission, she had become gradually less responsive. There had been no current medications or lifestyle changes, and she had not reported any specific symptoms before this event. Clinically she had no focal localising neurological signs or clinical evidence of infection. Blood tests showed within standard targets, electrolytes, and haemoglobin. There were no relevant changes on the electrocardiogram. An urgent CT head was performed that showed only a mature old right-sided infarction which the patient had in the year before this admission, with no acute changes. Her serum inflammatory markers were not elevated, and she was afebrile. She was treated empirically with intravenous vancomycin and gentamicin to cover for possible occult dialysis catheter-related infection. Subsequent culture results taken at this time all showed no microbial growth, and she remained drowsy despite antibiotic therapy for two days.
On day three of her admission, it was noted that her oral baclofen (10 mg three times per day) had not been given as it had not been available from the hospital pharmacy. At this time, she was noted to be becoming more responsive and even conversational. As her baclofen was reintroduced, she was found to become less responsive again. Due to this coincidence, baclofen was stopped. On day four, she did not tolerate a haemodialysis session due to persistent hypotension. In discussion with her family, withdrawal of dialysis was deemed appropriate with a switch to focusing on symptom management alone. However, with only a brief session of haemodialysis, it was recognised that if baclofen were the culprit, it would not have had the time to be completely cleared from her system. She had a trial of a full four-hour dialysis session. By the end of the session, she had become more alert. With ongoing dialysis sessions, she continued to become more responsive, and the muscle twitching resolved. She returned to her cognitive baseline and was eventually discharged home.
Discussion
Several causes can reduce the level of consciousness in haemodialysis patients. This list includes cerebrovascular Stroke, infections, hypoglycaemia, uraemic encephalopathy, hepatic encephalopathy (in dialysis cirrhotic patients), uncontrolled hypertension (PRES= posterior reversible encephalopathy syndrome), and thiamine deficiency. Another important cause to be considered in the absence of any other cause is drug-induced mental changes. Haemodialysis patients are more exposed to drug-related adverse effects and toxicity as they have reduced renal drug elimination in comparison to the general population [9].
Medications review in conjunction with a systematic approach can provide critical diagnostic pointers. In this case, medication review was the key to reaching the diagnosis, as Baclofen accumulation was the cause without any other apparent cause. Baclofen’s adverse reactions include hypotension, CNS depression and less frequent hypertonia. Hypotension complicates the situation as it affects dialysis and consequently affects drug elimination, as in this case. Chen et al.1997, described nine cases with renal impairment; 6 were on haemodialysis and presented with baclofen toxicity [5]. Also, Sue et al., 2009, presented a case of a haemodialysis lady who had impaired consciousness due to baclofen toxicity [10]. Muanda et al.2019, concluded that the 30-day encephalopathy risk was higher in older CKD patients prescribed higher baclofen doses (>20 mg/ day) in comparison with lower doses (<20 mg/day) [11]. Further analysis of this study, published in 2021, showed an increased risk of admissions with falls and hypotension [12].
In figure 1, Erin Wolf E et al showed a stepwise approach for baclofen dosing in CKD patients [8]. In patients with CKD, the Baclofen dose should be reduced even with mild CKD [13]. The results of the Maunda et al study and others suggest using baclofen with caution and in only low doses in older CKD patients on dialysis if highly indicated with close follow-up of signs of encephalopathy [11,14].
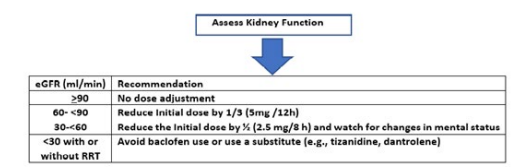
Figure 1: A stepwise approach for Baclofen dosing in patients with CKD, Modified from Erin Wolf E et al. [8].
Baclofen is dialysable and the main line of treatment for toxicity is drug removal by haemodialysis [6,7]. If toxicity is proven, haemodialysis can be used as a treatment option with the suspension of the medication. It may take a number of dialysis sessions to completely remove the medication. About 79% of the drug is removed in the first 4 hours dialysis session. Figure 2 shows baclofen drug removal over 2 dialysis sessions with suspending the drug [7].
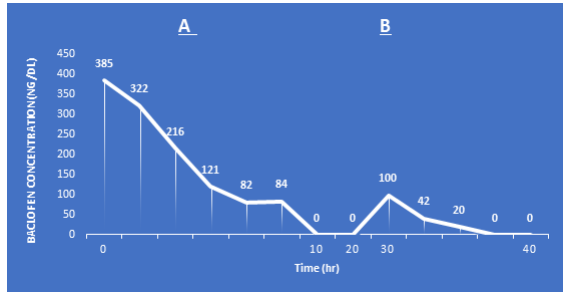
Figure 2: Changes of serum baclofen levels with time. A) The first haemodialysis session; B) second haemo-dialysis session. Modified from Wu VC et al. [7].
It is possible to measure serum baclofen levels, and this may be a viable approach for monitoring. There would be merited to check levels in haemodialysis patients when there is diagnostic uncertainty surrounding altered consciousness. Perhaps most importantly, alternative drugs can be used to treat spasticity, such as tizanidine and dantrolene [8].
Conclusion
Baclofen toxicity is an under-recognised phenomenon in haemodialysis patients and is an important differential diagnosis to consider in haemodialysis patients with reduced consciousness. Dialysis itself is a viable treatment option while safer alternative medications are sought. If baclofen is required it should be used with a reduced dose, increased time intervals between doses and close monitoring for signs of toxicity.
References
1. Romito, J.W., Turner, E.R., Rosener, J.A., Coldiron, L., Udipi, A., et al. (2021). Baclofen therapeutics, toxicity, and withdrawal: A narrative review. SAGE Open Med, 9, 20503121211022197.
2. Davidoff, R.A. (1985). Antispasticity drugs: mechanisms of action. Ann Neurol, 17(2), 107-116.
3. Ertzgaard, P., Campo, C., & Calabrese, A. (2017). Efficacy and safety of oral baclofen in the management of spasticity: A rationale for intrathecal baclofen. J Rehabilitation Med, 49(3), 193-203.
4. Wuis, E.W., Dirks, M.J., Termond, E.F., Vree, T.B., & Van der Kleijn, E. (1989). Plasma and urinary excretion kinetics of oral baclofen in healthy subjects. European J Clinical Pharmacol, 37(2), 181-184.
5. Chen, K.S., Bullard, M.J., Chien, Y.Y., & Lee, S.Y. (1997). Baclofen toxicity in patients with severely impaired renal function. The Annals of Pharmacotherapy, 31(11), 1315- 1320.
6. Meulendijks, D., Khan, S., Koks, C.H., Huitema, A.D., Schellens, J.H., & Beijnen, J.H. (2015). Baclofen overdose treated with continuous venovenous hemofiltration. European J Clinical Pharmacol, 71(3), 357-361.
7. Wu, V.C., Lin, S.L., Lin, S.M., & Fang, C.C. (2005). Treatment of baclofen overdose by haemodialysis: a pharmacokinetic study. Nephrol Dial Transplant, 20(2), 441-443.
8. Wolf, E., Kothari, N.R., Roberts, J.K., & Sparks, M.A. (2018). Baclofen Toxicity in Kidney Disease. Am J Kidney Dis, 71(2), 275–280.
9. Hae Ri, K., Kang, Wook L., Won Jung, C., Jae Wan, J., et al. (2019). Mental change in hemodialysis patients with end-stage renal disease without any abnormality in brain imaging. Nephrol Dialysis Transplantation, 34, gfz103. SP665. 10. Su, W., Yegappan, C., Carlisle, E.J., & Clase, C.M. (2009). Reduced level of consciousness from baclofen in people with low kidney function. BMJ (Clinical research ed.), 339, b4559.
11. Muanda, F.T., Weir, M.A., Bathini, L., Blake, P.G., Chauvin, K., et al. (2019). Association of Baclofen With Encephalopathy in Patients With Chronic Kidney Disease. JAMA, 322(20), 1987–1995.
12. Muanda, F.T., Blake, P.G., Weir, M.A., Bathini, L., Chauvin, K., Dixon, S.N., et al. (2021). Association of Baclofen With Falls and Fractures in Patients With CKD. Am J Kidney Dis, 78(3), 470-473. 13. Brunton, L.L., Chabner, B.A., Knollmann, B.C. (2011). Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. 12th Edition, McGraw-Hill, New York, 883- 884.
14. Chauvin, K.J., Blake, P.G., Garg, A.X., Weir, M.A., Bathini, L., et al. (2020). Baclofen has a risk of encephalopathy in older adults receiving dialysis. Kidney Int, 98(4), 979-988.
Copyright: © 2025 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



